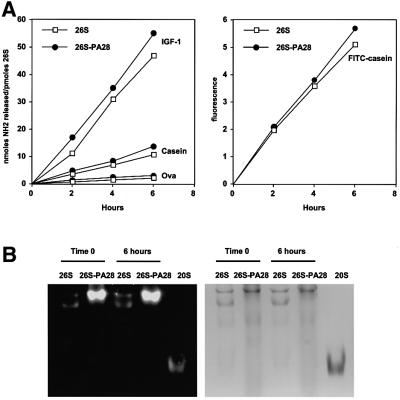
Fig. 3. (A) Association of PA28 with the 26S proteasomes does not increase the rate of protein breakdown. Degradation of reductively methylated IGF-1 (1.5 mM), casein (1 mM), ovalbumin (10 µM) and FITC–casein (10 µM) was performed at 37°C in 20 mM Bis-Tris-propane pH 7.5, 2 mM ATP, 5 mM MgCl2, 1 mM EDTA with a concentration of the enzyme variable between 10 and 30 nM depending on the substrate. At the indicated times, aliquots were removed and analyzed for the appearance of amino groups with fluorescamine (for IGF-1, casein and ovalbumin) or for the generation of soluble fluorescence after precipitation with perchloric acid (for FITC–casein). (B) Even after a 6 h incubation at 37°C, only 26S and hybrid complexes could be detected in the digestion assays. 26S and 26S–PA28 were incubated for 6 h in the presence of casein. At time 0 and after 6 h, an aliquot was removed and loaded onto a native polyacrylamide gel. The gel was overlaid with fluorogenic substrate, and then Coomassie Blue stained. Even after 6 h, 26S particles (5 µg) in the presence of PA28 were several fold more active than control 26S alone, and no 20S particles could be detected.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.