Abstract
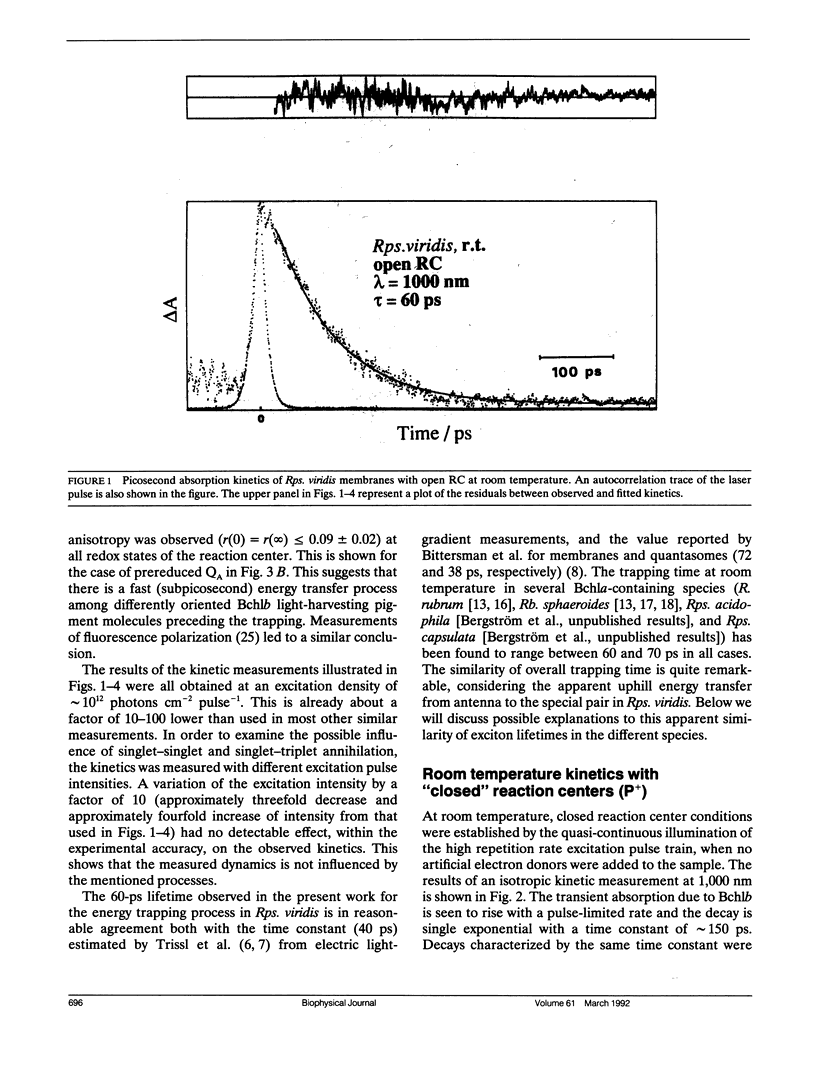
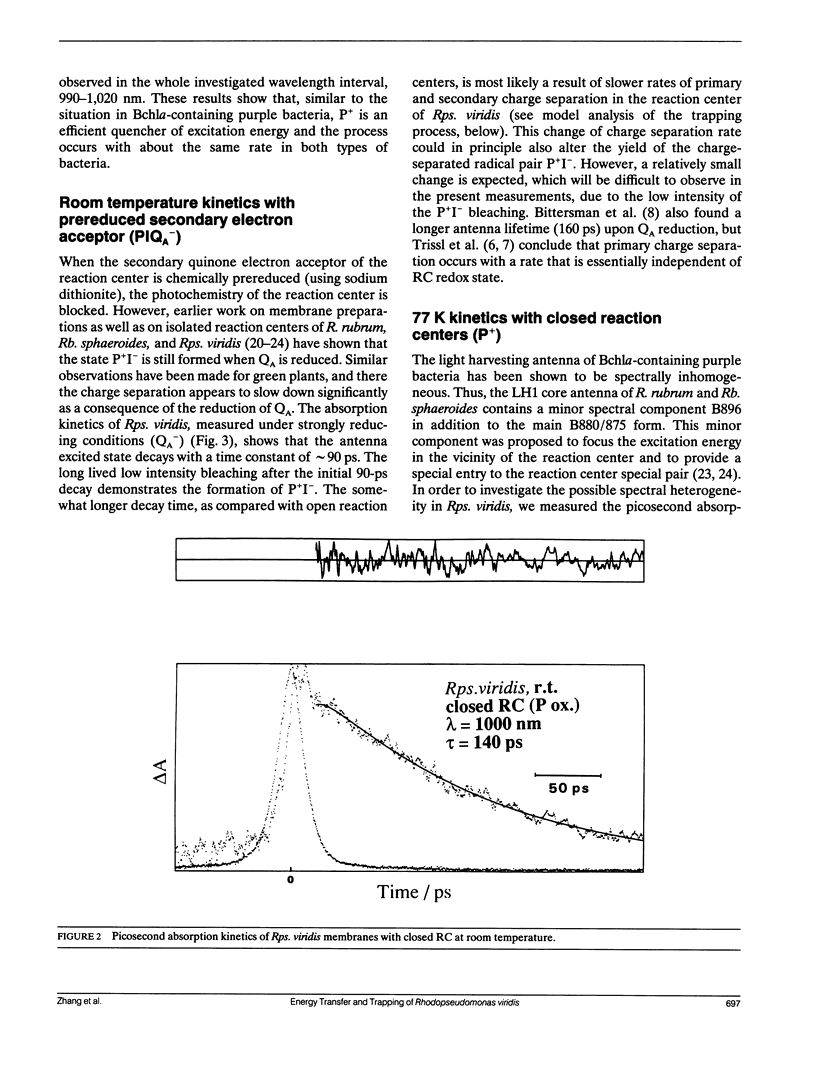
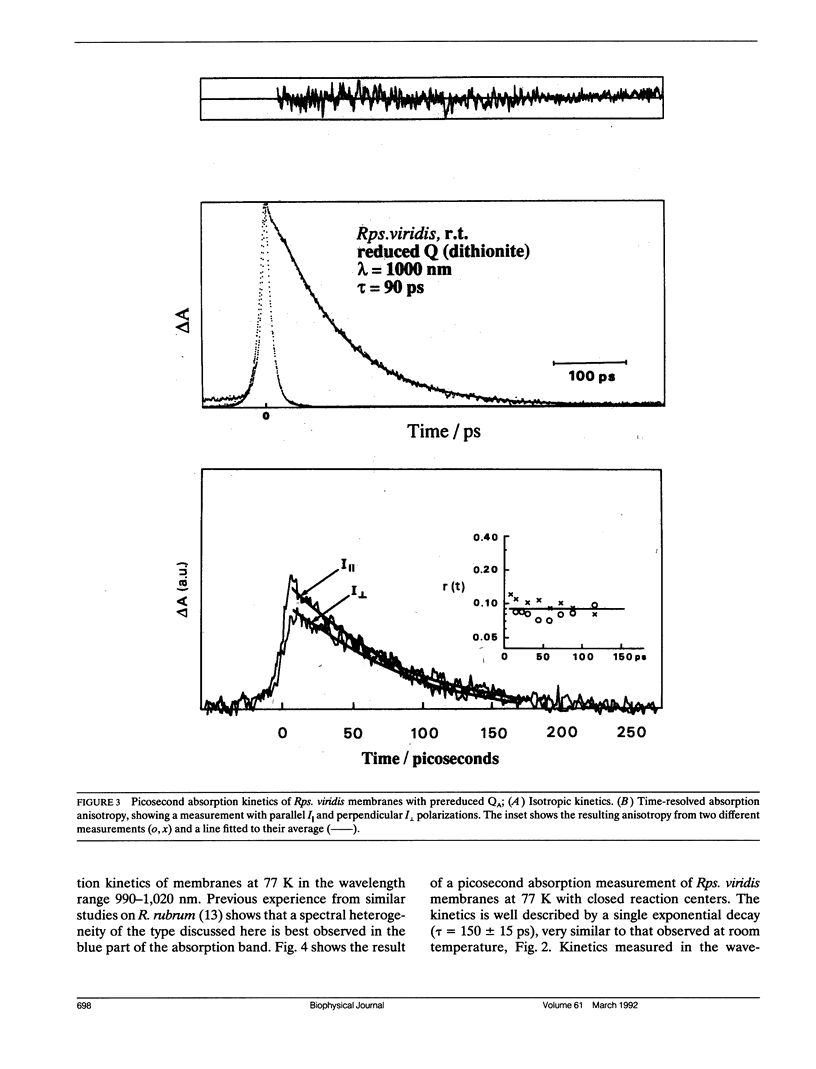
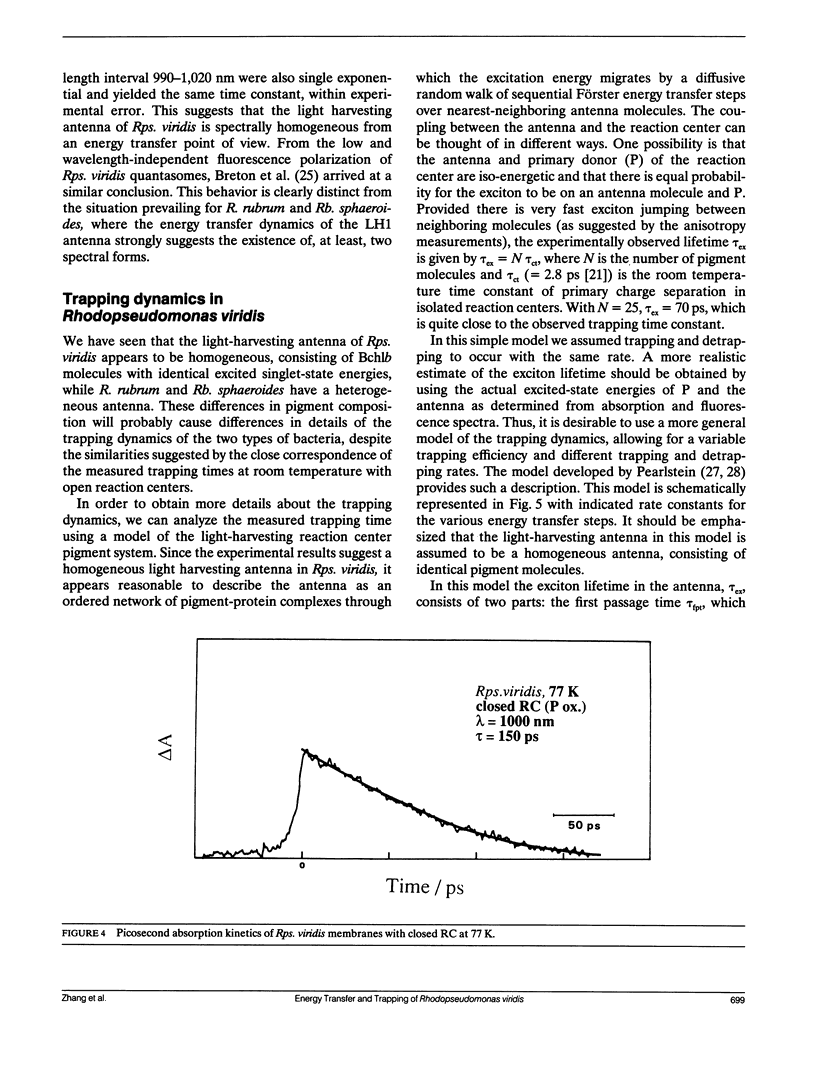
By low intensity picosecond absorption spectroscopy it is shown that the exciton lifetime in the light-harvesting antenna of Rhodopseudomonas (Rps.) viridis membranes with photochemically active reaction centers at room temperature is 60 +/- 10 ps. This lifetime reflects the overall trapping rate of the excitation energy by the reaction center. With photochemically inactive reaction centers, in the presence of P+, the exciton lifetime increases to 150 +/- 15 ps. Prereducing the secondary electron acceptor QA does not prevent primary charge separation, but slows it down from 60 to 90 +/- 10 ps. Picosecond kinetics measured at 77 K with inactive reaction centers indicates that the light-harvesting antenna is spectrally homogeneous. Picosecond absorption anisotropy measurements show that energy transfer between identical Bchlb molecules occurs on the subpicosecond time scale. Using these experimental results as input to a random-walk model, results in strict requirements for the antenna-RC coupling. The model analysis prescribes fast trapping (approximately 1 ps) and an approximately 0.5 escape probability from the reaction center, which requires a more tightly coupled RC and antenna, as compared with the Bchla-containing bacteria Rhodospirillum (R.) rubrum and Rhodobacter (Rb.) sphaeroides.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breton J., Martin J. L., Migus A., Antonetti A., Orszag A. Femtosecond spectroscopy of excitation energy transfer and initial charge separation in the reaction center of the photosynthetic bacterium Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5121–5125. doi: 10.1073/pnas.83.14.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers R. P., Parson W. W. Delayed fluorescence from Rhodopseudomonas viridis following single flashes. Biochim Biophys Acta. 1975 May 15;387(2):194–211. doi: 10.1016/0005-2728(75)90103-6. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Deprez J., Trissl H. W., Breton J. Excitation trapping and primary charge stabilization in Rhodopseudomonas viridis cells, measured electrically with picosecond resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1699–1703. doi: 10.1073/pnas.83.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt A. S., Clayton R. K. Light-induced absorbancy changes in Eimhjellen's Rhodopseudomonas. Photochem Photobiol. 1965 Sep;4(4):829–831. doi: 10.1111/j.1751-1097.1965.tb07925.x. [DOI] [PubMed] [Google Scholar]
- Holten D., Windsor M. W., Parson W. W., Thornber J. P. Primary photochemical processes in isolated reaction centers of Rhodopseudomonas viridis. Biochim Biophys Acta. 1978 Jan 11;501(1):112–126. doi: 10.1016/0005-2728(78)90100-7. [DOI] [PubMed] [Google Scholar]
- Joliot P., Verméglio A., Joliot A. Electron transfer between primary and secondary donors in Rhodospirillum rubrum: evidence for a dimeric association of reaction centers. Biochemistry. 1990 May 8;29(18):4355–4361. doi: 10.1021/bi00470a014. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillotin G., Swenberg C. E., Breton J., Geacintov N. E. Analysis of picosecond laser induced fluorescence phenomena in photosynthetic membranes utilizing a master equation approach. Biophys J. 1979 Mar;25(3):513–533. doi: 10.1016/S0006-3495(79)85320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W. Photosynthetic bacterial reaction centers: interactions among the bacteriochlorophylls and bacteriopheophytins. Annu Rev Biophys Bioeng. 1982;11:57–80. doi: 10.1146/annurev.bb.11.060182.000421. [DOI] [PubMed] [Google Scholar]
- Stark W., Kühlbrandt W., Wildhaber I., Wehrli E., Mühlethaler K. The structure of the photoreceptor unit of Rhodopseudomonas viridis. EMBO J. 1984 Apr;3(4):777–783. doi: 10.1002/j.1460-2075.1984.tb01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]