Abstract
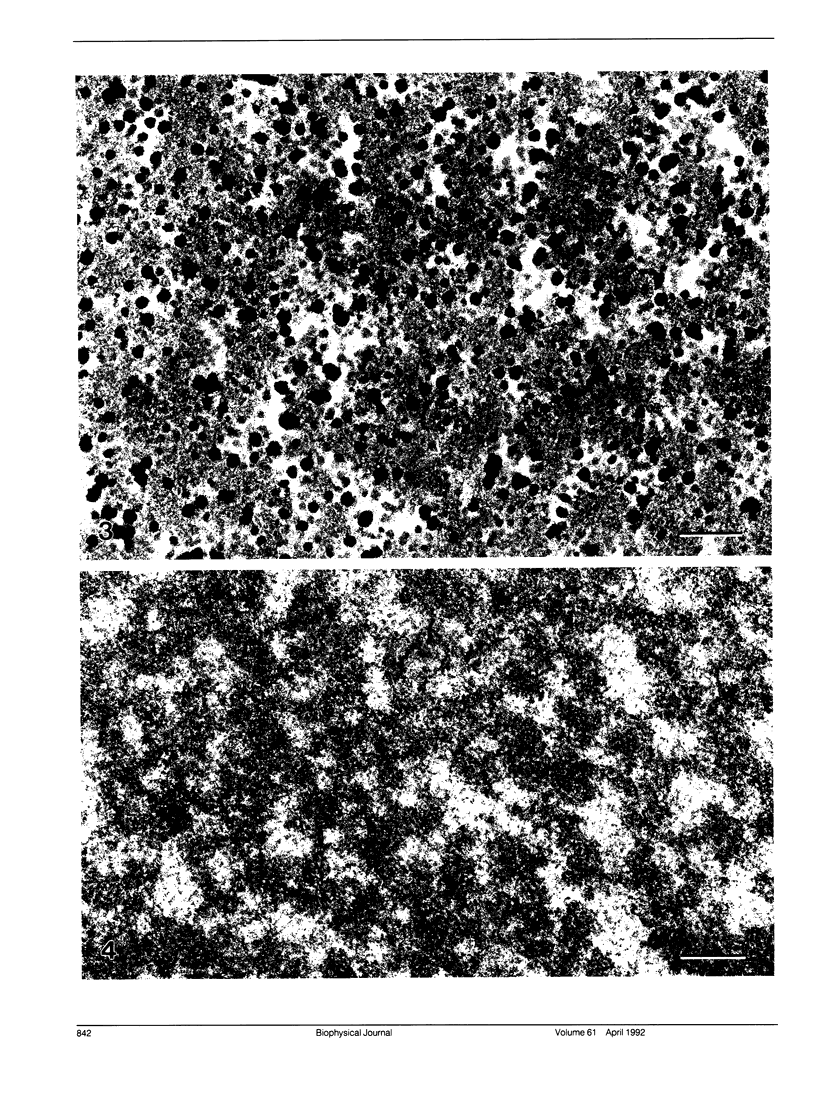
Electron microscopy confirms the presence of a high concentration of glycogen particles in the lens nuclear region of birds of flying habit such as the ring-neck dove and pigeon. This observation is consistent with Raman spectroscopy. The glycogen particles in the dove lens, which are approximately 35 nm in diameter, are classified as beta type particles. Although this type has been previously characterized by high rates of glycogen turnover in other tissues, its localization in the lens nucleus indicates that it may serve a structural function rather than as a storage depot of carbohydrate in the lens. In a comparative electron microscopy study, glycogen particles were not observed in the chicken lens.
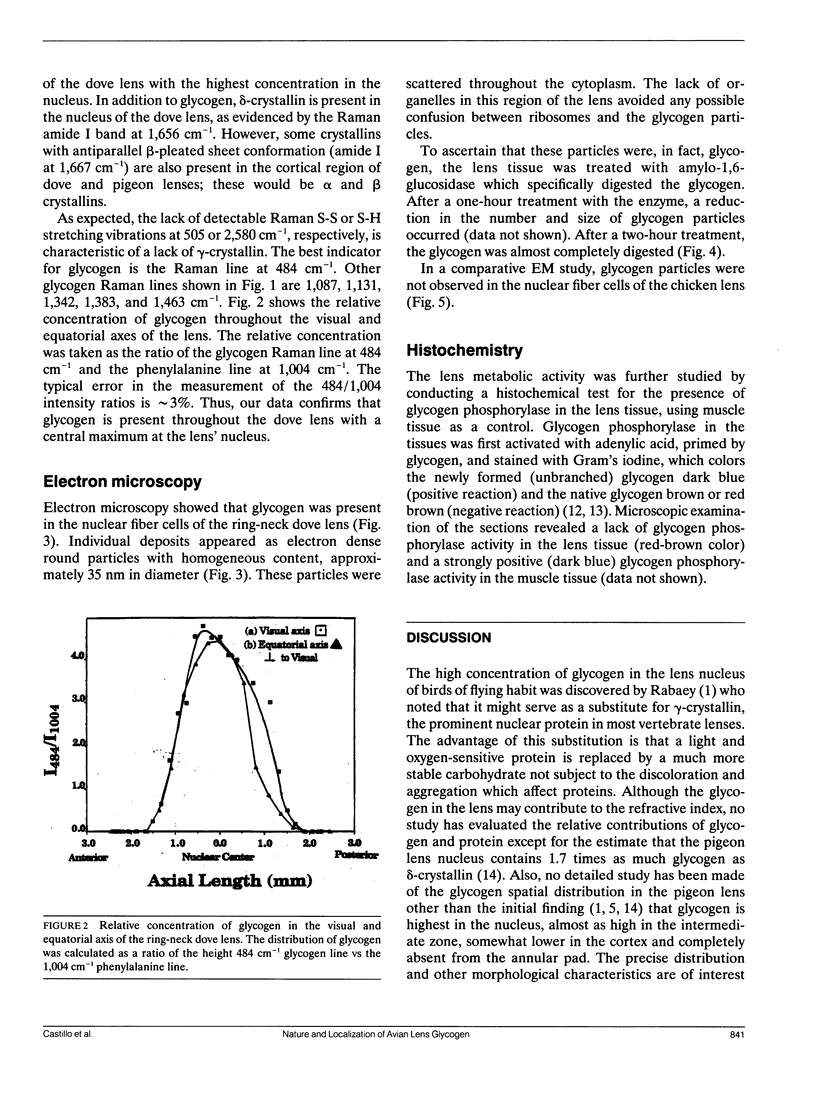
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Duvall A. J., 3rd, Hukee M. J. Delineation of cochlear glycogen by electron microscopy. Ann Otol Rhinol Laryngol. 1976 Mar-Apr;85(2 PT1):234–246. doi: 10.1177/000348947608500208. [DOI] [PubMed] [Google Scholar]
- Hockwin O. The presence of glycogen in lenses of different species. Exp Eye Res. 1973 Feb;15(2):235–244. doi: 10.1016/0014-4835(73)90124-3. [DOI] [PubMed] [Google Scholar]
- Kuck J. F., Jr, East E. J., Yu N. T. Prevalence of alpha-helical form in avian lens proteins. Exp Eye Res. 1976 Jul;23(1):9–14. doi: 10.1016/0014-4835(76)90023-3. [DOI] [PubMed] [Google Scholar]
- LUMSDEN R. D. MACROMOLECULAR STRUCTURE OF GLYCOGEN IN SOME CYCLOPHYLLIDEAN AND TRYPANORHYNCH CESTODES. J Parasitol. 1965 Aug;51:501–515. [PubMed] [Google Scholar]
- Lo W. K. Adherens junctions in the ocular lens of various species: ultrastructural analysis with an improved fixation. Cell Tissue Res. 1988 Oct;254(1):31–40. doi: 10.1007/BF00220014. [DOI] [PubMed] [Google Scholar]
- MANNERS D. J. The molecular structure of glycogens. Adv Carbohydr Chem. 1957;12:261–298. doi: 10.1016/s0096-5332(08)60210-6. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Rogers K. J. An enzymic fluorometric micro method for determination of glycoen. Anal Biochem. 1972 Oct;49(2):492–497. doi: 10.1016/0003-2697(72)90453-8. [DOI] [PubMed] [Google Scholar]
- RABAEY M. Glycogen in the lens of birds' eyes. Nature. 1963 Apr 13;198:206–207. doi: 10.1038/198206a0. [DOI] [PubMed] [Google Scholar]
- Ryman B. E., Whelan W. J. New aspects of glycogen metabolism. Adv Enzymol Relat Areas Mol Biol. 1971;34:285–443. doi: 10.1002/9780470122792.ch6. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T., KURIAKI H. Histochemical detection of phosphorylase in animal tissues. J Histochem Cytochem. 1955 May;3(3):153–160. doi: 10.1177/3.3.153. [DOI] [PubMed] [Google Scholar]
- Yu N. T., East E. J., Chang R. C., Kuck J. F. Raman spectra of bird and reptile lens proteins. Exp Eye Res. 1977 Apr;24(4):321–334. doi: 10.1016/0014-4835(77)90145-2. [DOI] [PubMed] [Google Scholar]





