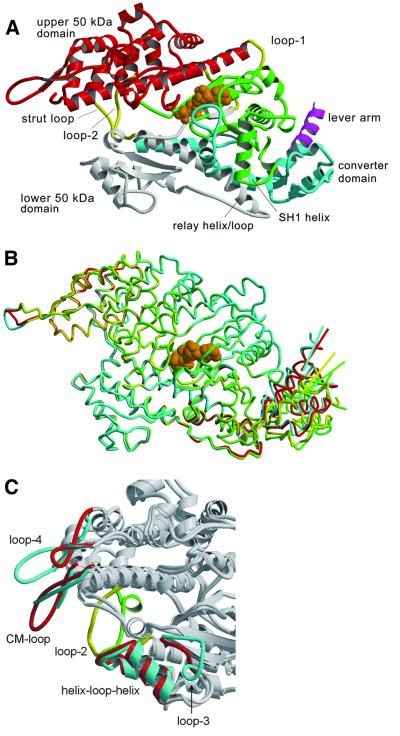
Fig. 1. Structure of the motor momain of the class-I myosin MyoE from D.discoideum. (A) Ribbon diagram of MyoE, colored according to structural domains. The N-terminus (green) lacks the SH3-like domain present in conventional myosin, but otherwise has a similar fold. It contains part of the nucleotide-binding site and leads into the upper 50 kDa domain (red). The lower 50 kDa domain (white) contains the main actin-binding motifs, switch-2 and the relay region. The C-terminal domain (cyan) consists of the converter domain, the beginning of the lever arm helix (magenta), and a long α-helix connecting the converter to loop-2 in the actin-binding site. The transition state analog MgADP·VO4 is shown in orange, and loop-1, loop-2 and the strut loop are shown in yellow. (B) Superposition of the four molecules in the asymmetric unit shows good overlay in the core regions. The relay helices, adjacent relay loops and converter domains are in different positions. Colored lines indicate the trajectory of the lever arm helix as it leaves the converter domain for each molecule. (C) Superposition of MyoE and the Dictyostelium myosin II structures shows the differences in the actin-binding loops (MyoE, cyan and yellow; DdMyoII, red and green). All figures were produced using Molscript (Kraulis, 1991) and Raster3d (Merritt and Bacon, 1997).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.