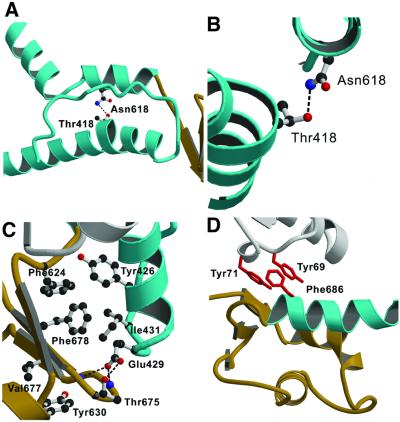
Fig. 3. Contacts between the relay region, converter domain and lever arm helix allow these structural elements to move together. (A) The relay region and SH1 helix of MyoE are shown in cyan. In MyoE and other class-I myosins, there is a hydrogen bond between Thr418 in the relay helix and Asn618 from the SH1 helix. (B) Close-up view of this region, viewed along the relay helix. The kink forms at Thr418. (C) Highly conserved residues form a hydrophobic core, and polar residues further stabilize the link via conserved hydrogen bonds (dashed lines). This core interaction is further supported by a small, hydrophobic, highly conserved extension into the converter formed by residues Tyr630 and Val677 (DdTyr699 and DdIle744). At the tip of the relay loop (cyan), conserved Glu429 (DdGlu497) forms hydrogen bonds to residue Thr675 of the converter domain (brown) (DdThr742; at this position there is always a threonine or a serine) and to the backbone nitrogen atoms of converter residues Lys674 and Lys676. (D) Hydrophobic interactions between the lever arm helix (cyan) and core domain (white). All class-I myosins contain an aromatic residue at the positions of Tyr69 and/or Tyr71 (red) in close contact with the conserved Phe686 (red) in the lever arm helix. Either a glycine or an alanine is found at the equivalent position to Phe686 in class-II myosins.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.