Abstract
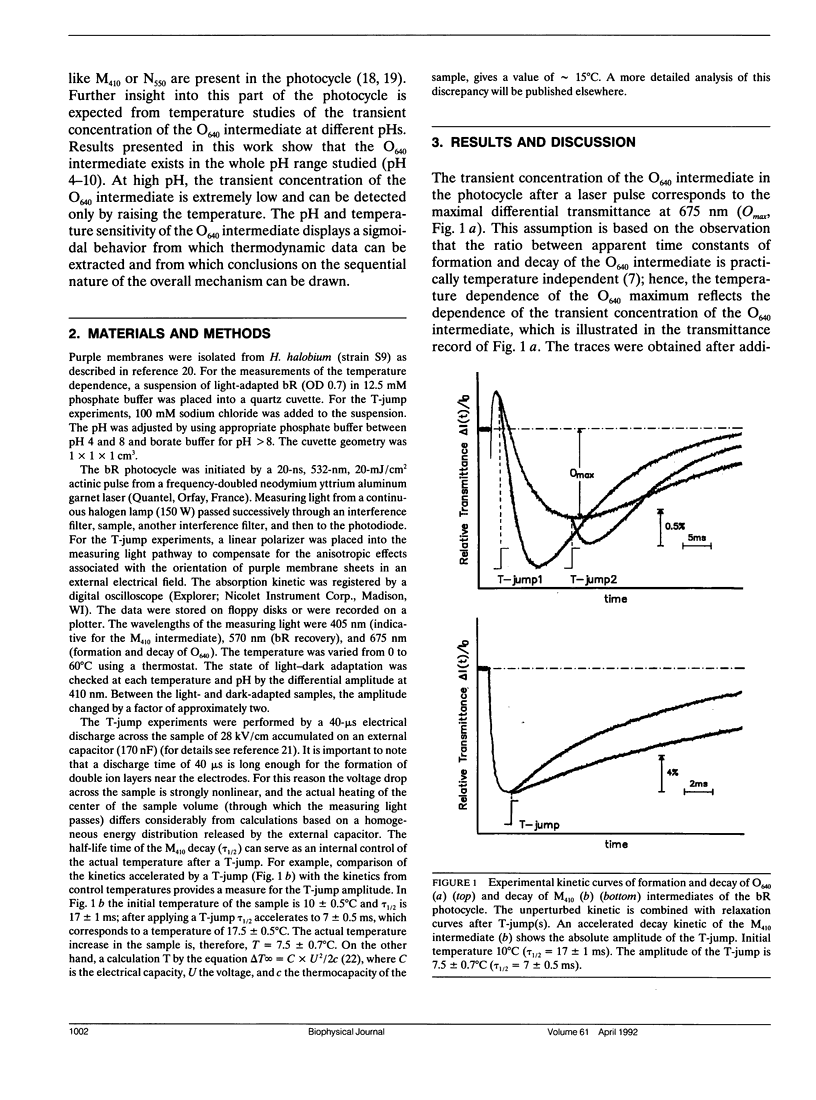
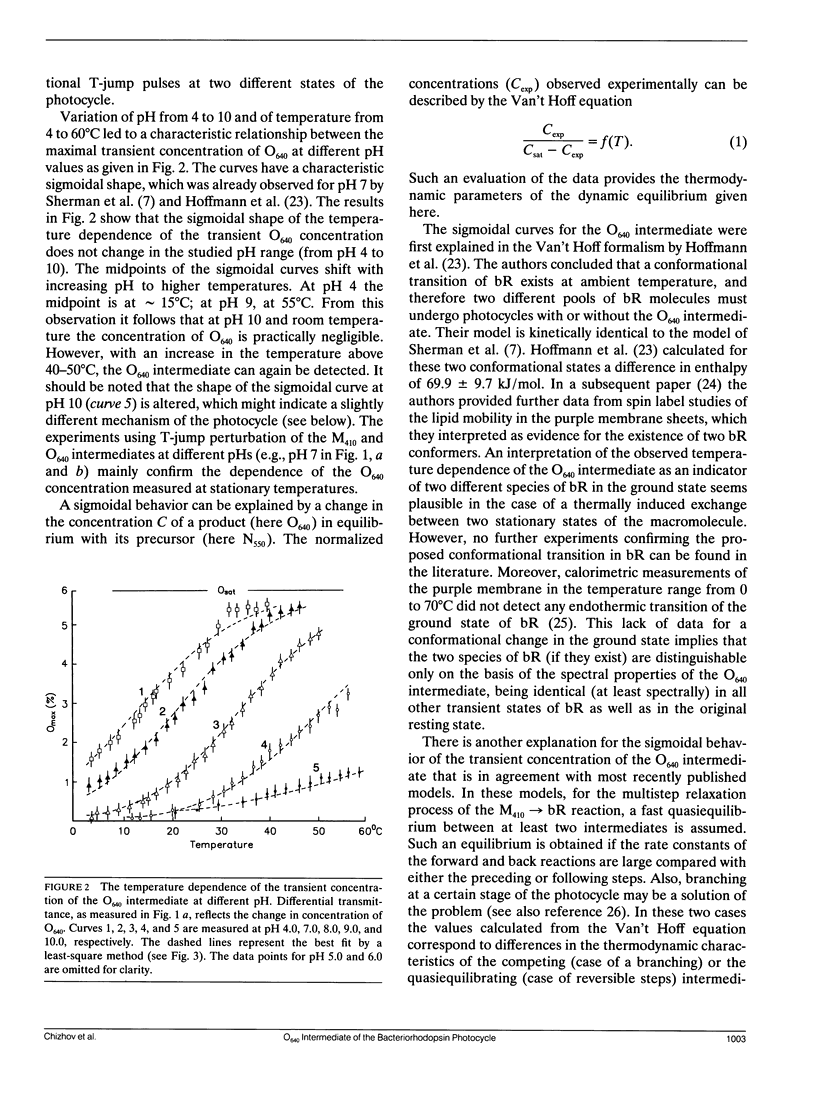
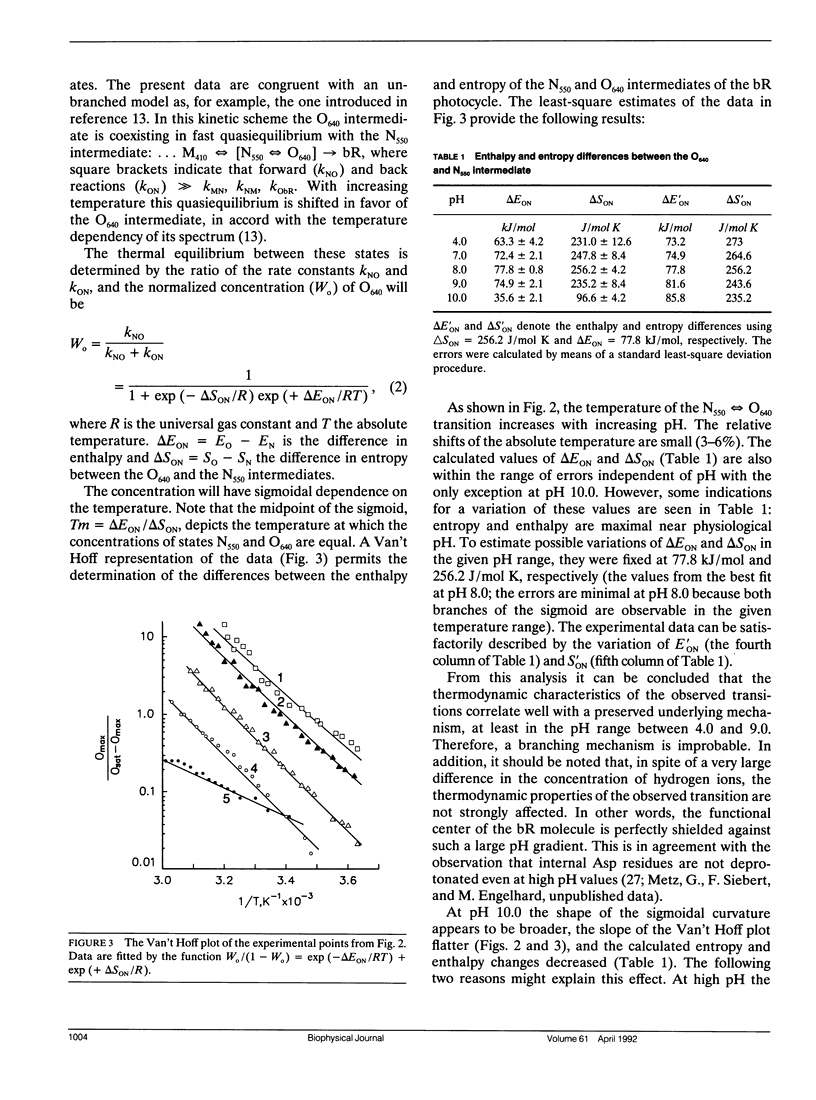
The temperature and pH dependencies of the O640 intermediate of the photocycle of bacteriorhodopsin (bR) were investigated by flash photolysis and T-jump experiments. The maximal concentration of the O640 intermediate was found to be dependent on the temperature, which is described by a sigmoidal relationship. With increasing pH the midpoint of the sigmoidal curves shifts to higher temperatures. The Van't Hoff equation provides enthalpy and entropy values of the observed states. These results indicate that, in the investigated temperature (0-60°C) and pH (pH 4.0-10.0) range, the sequence of the principal intermediates in the pathway “M-N-O-bR” does not change. The observations of the O640 intermediate at pH < 8.0 and of the N550 intermediate at pH > 8.0 are most probably due only to changes of the intrinsic rate constants of the bR photocycle, not to a different mechanism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Chernavskii D. S., Chizhov I. V., Lozier R. H., Murina T. M., Prokhorov A. M., Zubov B. V. Kinetic model of bacteriorhodopsin photocycle: pathway from M state to bR. Photochem Photobiol. 1989 May;49(5):649–653. doi: 10.1111/j.1751-1097.1989.tb08437.x. [DOI] [PubMed] [Google Scholar]
- Chernavskii D. S., Khurgin Iu I., Shnol' S. E. Kontseptsiia "belok-mashina" i ee sledstviia. Biofizika. 1987 Sep-Oct;32(5):775–781. [PubMed] [Google Scholar]
- Chu Kung M., DeVault D., Hess B., Oesterhelt D. Photolysis of bacterial rhodopsin. Biophys J. 1975 Sep;15(9):907–911. doi: 10.1016/S0006-3495(75)85864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M., Bakker E. P., Korenstein R., Caplan S. R. Bacteriorhodopsin: biphasic kinetics of phototransients and of light-induced proton transfer by sub-bacterial Halobacterium halobium particles and by reconstituted liposomes. FEBS Lett. 1976 Dec 1;71(2):228–232. doi: 10.1016/0014-5793(76)80938-6. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Groma G. I., Dancshazy Z. How Many M Forms are there in the Bacteriorhodopsin Photocycle? Biophys J. 1986 Aug;50(2):357–366. doi: 10.1016/S0006-3495(86)83469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Clark A. D., Turner M., Wyard S., Chapman D. Bacteriorhodopsin, boundary lipid and protein conformers: a spin label study. Biochim Biophys Acta. 1980 May 8;598(1):178–183. doi: 10.1016/0005-2736(80)90276-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Graca-Miguel M., Barnard P., Chapman D. Evidence for conformational transitions in bacteriorhodopsin. FEBS Lett. 1978 Nov 1;95(1):31–34. doi: 10.1016/0014-5793(78)80045-3. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Sturtevant J. M. Phase transitions of the purple membranes of Halobacterium halobium. Biochemistry. 1978 Mar 7;17(5):911–915. doi: 10.1021/bi00598a026. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B., Kuschmitz D. Branching reactions in the photocycle of bacteriorhodopsin. FEBS Lett. 1978 Sep 15;93(2):266–270. doi: 10.1016/0014-5793(78)81118-1. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Enthalpy changes during the photochemical cycle of bacteriorhodopsin. Biophys J. 1979 Feb;25(2 Pt 1):355–364. doi: 10.1016/s0006-3495(79)85297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Modyanov N. N. Structural basis of proton-translocating protein function. Annu Rev Biophys Bioeng. 1982;11:445–463. doi: 10.1146/annurev.bb.11.060182.002305. [DOI] [PubMed] [Google Scholar]
- Parodi L. A., Lozier R. H., Bhattacharjee S. M., Nagle J. F. Testing kinetic models for the bacteriorhodopsin photocycle--II. Inclusion of an O to M backreaction. Photochem Photobiol. 1984 Oct;40(4):501–506. doi: 10.1111/j.1751-1097.1984.tb04624.x. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Váró G., Duschl A., Lanyi J. K. Interconversions of the M, N, and O intermediates in the bacteriorhodopsin photocycle. Biochemistry. 1990 Apr 17;29(15):3798–3804. doi: 10.1021/bi00467a029. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Pathways of the rise and decay of the M photointermediate(s) of bacteriorhodopsin. Biochemistry. 1990 Mar 6;29(9):2241–2250. doi: 10.1021/bi00461a006. [DOI] [PubMed] [Google Scholar]


