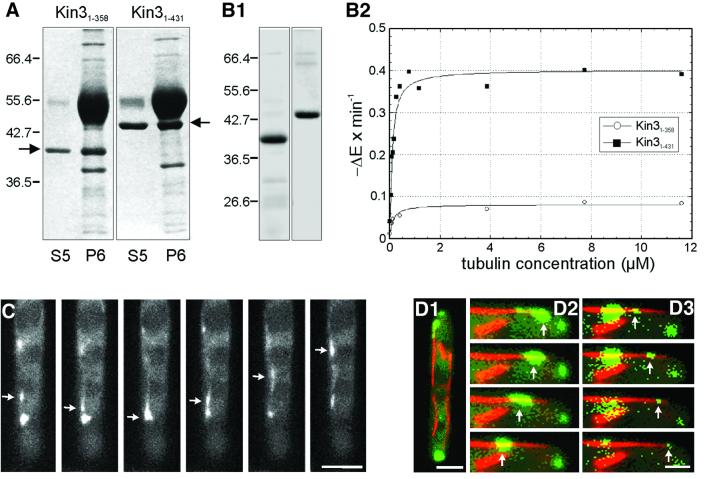
Fig. 2. MT affinity, ATPase activity and in vivo motility of Kin3. (A) MT affinity of recombinant Kin3 heads. After ATP depletion, bacterial expressed Kin3 motor domains bound to taxol-stabilized MTs, and were partially released by MgATP (S5), while some protein was sedimented with MTs (P6). Kin31–358, amino acids 1–358; Kin31–431, amino acids 1–431 of Kin3 (arrows). (B) His-tagged Kin3 was affinity purified (B1) and ATPase activity, shown as NADH reduction per time in the presence of increasing tubulin concentrations, was measured (B2). Note that the neck region (amino acids 358–431) is required for MT stimulation of truncated motor domain. (C) Movement of Kin3–GFP. The Kin3–GFP fusion protein (arrow) moves rapidly in a bidirectional fashion. Time between frames is 500 ms. Bar: 3 µm. (D) Co-localization of Kin3–YFP- and CFP–Tub1-marked microtubules. MTs are running through the length of an unbudded cell (D1). A series of images of Kin3–YFP (green, arrows) was taken and merged with a single image of CFP–Tub1 (red). Movement of a large accumulation of Kin3–YFP (D2), as well as of small Kin3–YFP-labeled dots (D3) along MTs. Time between frames is 700 ms. Bar: 2 µm (D1) and 1 µm (D2 and D3). A movie for this figure is available as Supplementary data at The EMBO Journal Online.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.