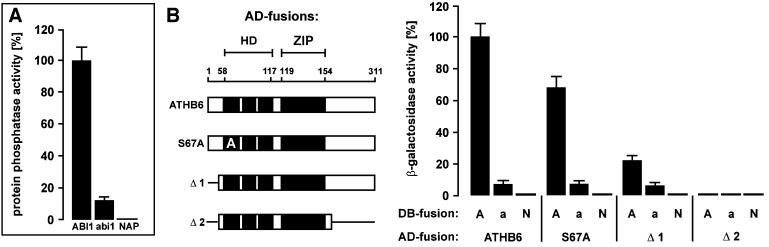
Fig. 2. Dependence of ABI1 and ATHB6 interaction on the functional catalytic domain of PP2C. (A) Specific protein phosphatase activity of purified ABI1, and both point-mutated forms abi1 (ABI1Gly180Asp) and non-active ABI1Asp177Ala (NAP) were measured and expressed as relative activities (n = 3, ± SD). (B) The homeodomain (HD) and leucine zipper (ZIP) regions of the AD fusion for ATHB6 (amino acids 1–311) and mutant versions thereof [Δ1 (amino acids 44–311), Δ2 (amino acids 44–217) and S67A (ATHB6Ser67Ala)] are presented schematically (left panel). They were analysed for binding to DB fusions of ABI1 (A), abi1 (a) and NAP (N) in the yeast two-hybrid system. The β-galactosidase activities for combination with the empty AD vector were subtracted as background from the values presented.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.