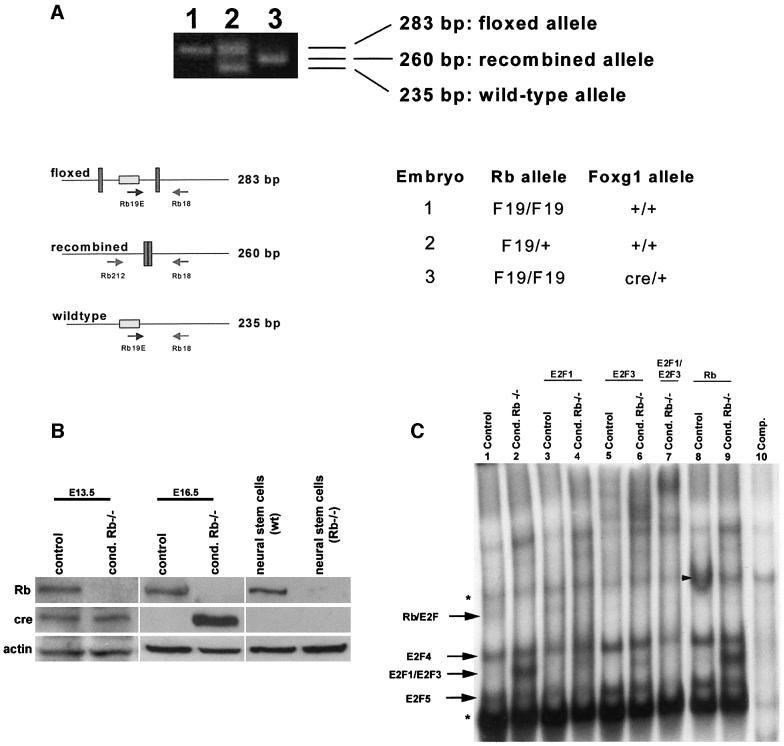
Fig. 2. Rb is completely deleted from the telencephalon of conditional mutants. (A) To detect the presence of Rb exon 19, DNA isolated from the telencephalon of E16.5 mutant and control embryos was subjected to PCR analysis. In the absence of cre, the floxed and wild-type alleles remain intact, as determined by 283 and 235 bp fragments, respectively. When cre-mediated recombination occurs, the floxed allele is deleted, resulting in a 260 bp band (Marino et al., 2000). The conditional mutant (embryo 3) exhibited only the middle 260 bp band, indicating that recombination had occurred, with no detection of the intact floxed allele (n = 3). (B) Rb and cre protein expression were assayed at E13.5 and E16.5 in tissue isolated from the telencephalon of mutant (homozygous Rb-F19 and heterozygous for Foxg1-cre) and control (double heterozygous at E13.5 and heterozygous for Rb-F19 at E16.5) embryos. As control, protein was also assayed from neural stem cells derived from wild-type and germline Rb–/– brains. Rb protein was undetectable in the mutant telencephalon (n = 4). (C) Protein was extracted from the telencephalon of E16.5 mutant and control littermates and subjected to EMSA. In contrast to the control (lane 1), the mutant extract exhibited increased levels of free E2F1/3 (lane 2). The uncomplexed E2F band was partially deleted upon the addition of an antibody to E2F1 (lane 4) or supershifted with antibodies specific for E2F3 (lane 6), and was completely deleted when antibodies to both E2F1 and E2F3 were added (lane 7). In the mutant extract, Rb–E2F complexes were undetectable (lane 2). The addition of the Rb antibody produced a supershift in littermate controls (arrowhead, lane 8) but not in extracts from the conditional Rb mutant (lane 9). Asterisks denote non-specific bands (n = 4).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.