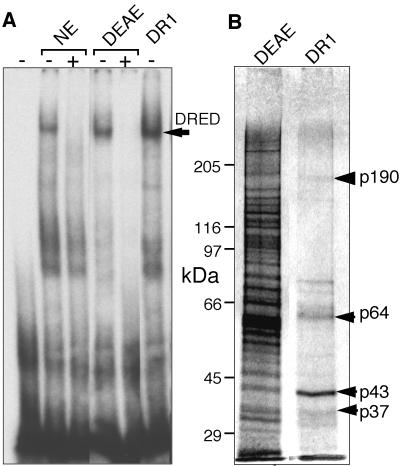
Fig. 3. DRED purification. The DRED complex was purified as described in Materials and methods. (A) EMSA was performed by incubating crude nuclear extract (NE; 2 µg of protein), the pooled DEAE Sepharose fraction (DEAE, 0.2 µg, 10 µl) or the peak fraction from the DNA sequence affinity column (DR1, 10 µl) with radiolabeled ‘epsi’ probe with (+) or without (–) preincubation with excess unlabeled probe. The arrow indicates the mobility of the DRED complex. (B) Proteins recovered in the peaks of the DEAE Sepharose and DR1 sequence affinity fractions (40 µl each) were separated by SDS–PAGE and silver-staining.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.