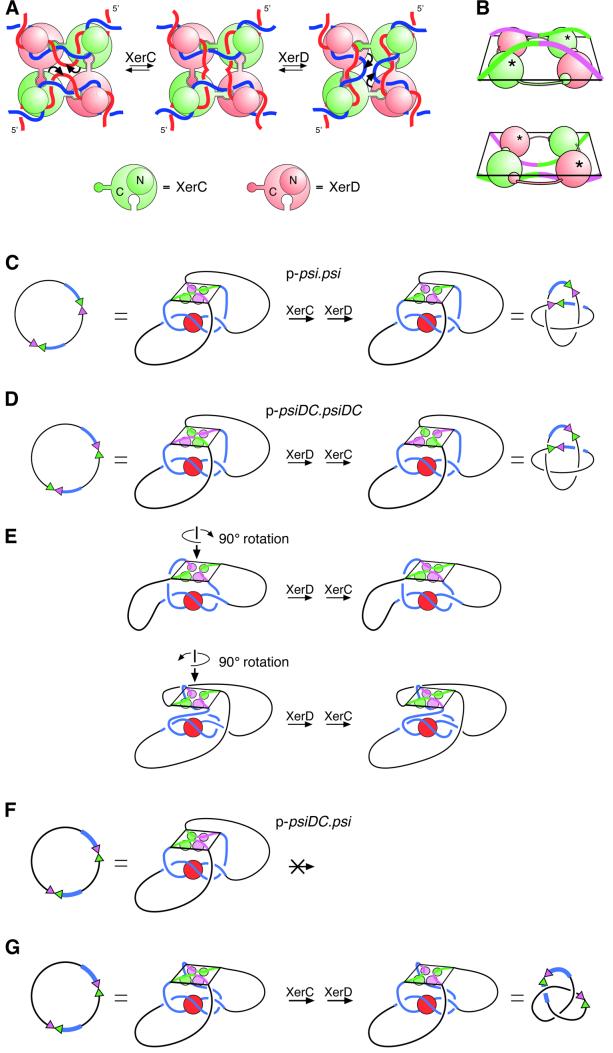
Fig. 7. Models for recombination at psi and psiDC. (A) The proposed mechanism of strand exchange by XerC and XerD at the psi core, based on X-ray crystal structures of the Cre recombinase bound to its recombination site. XerC (green) and XerD (pink) bind to a pair of psi core sites in an antiparallel conformation. A C-terminal extension on each monomer binds to the C-terminal domain of one of its neighbours in a cyclical arrangement. All four monomers bind to the DNA with their N-terminal domains (shown as small circles above the DNA) on one side of the HJ and their C-terminal domains (shown as large circles below the DNA) on the other side. XerC catalyses cleavage, strand exchange and re-ligation of one pair of strands (denoted by curly arrows) to form a HJ intermediate. XerD then exchanges the other pair of strands to form the recombinant product. Recombination can also occur by the reverse of this process (right to left) with XerD strand exchange preceding XerC strand exchange. (B) Perspective view of core–recombinase complexes in which XerC (top panel; equivalent to the left panel in A) or XerD (bottom panel; equivalent to the right panel in A) is about to catalyse strand exchange to form a HJ. The diagrams have been aligned so that DNA connectivity and the topological outcome of a complete strand exchange reaction will be the same in both cases. In this view, C-terminal domains have to be below the DNA for XerC strand exchange to proceed first, and above the DNA for XerD strand exchange to proceed first. XerC and XerD C-terminal domains are shown as green and pink spheres, respectively. N-terminal domains have been omitted for clarity. Active recombinase monomers are indicated with asterisks. (C) Synapsis of psi sites and interwrapping of accessory sequences around PepA define the order of strand exchange by XerC and XerD. During recombination between two psi sites, the accessory sequences (blue) of two psi sites wrap around PepA, with the psi cores brought together in an antiparallel fashion. XerC protein and binding sites are shown in green, and XerD protein and binding sites are shown in pink. This synapse is equivalent to that shown in Figure 1A. The recombinase–core complex is constrained so that the C-termini face towards PepA and the accessory sequences. By comparison with (A), it can be seen that the psi cores have been brought together such that XerC strand exchange will precede XerD strand exchange. The product is a four-noded catenane. (D) Two psiDC sites can form a synapse in which the DNA follows exactly the same path as in (C). In this complex, XerC occupies the position normally occupied by XerD, and vice versa. XerD strand exchange proceeds first in this synapse, and the product is a four-noded catenane. (E) The synapse shown in (D) can be rearranged into two geometrically different, but topologically equivalent synapses, by rotating the recombinase–core complex by ±90° about its 2-fold symmetry axis. XerC and XerD occupy the same positions in this synapse as in the synapse between wild-type psi sites, but the DNA path is altered to accommodate the psiDC sites. XerD strand exchange will proceed first and the product will be the observed four-noded catenane in both cases. (F) On p-psiDC.psi, interwrapping of accessory sequences as in (A) would bring the two cores together in a parallel conformation with forbidden XerC–XerD interactions. (G) To bring the two sites together in an antiparallel fashion, one of the cores must be rotated through 180° relative to its position in (F). If the core of psiDC is rotated, as depicted, XerC strand exchange will proceed first and the product will be a three-noded knot, as observed. However, an equivalent rotation of the psi core would give the same three-noded product but XerD strand exchange would proceed first. Alternatively, both cores might be rearranged to bring about the required antiparallel alignment.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.