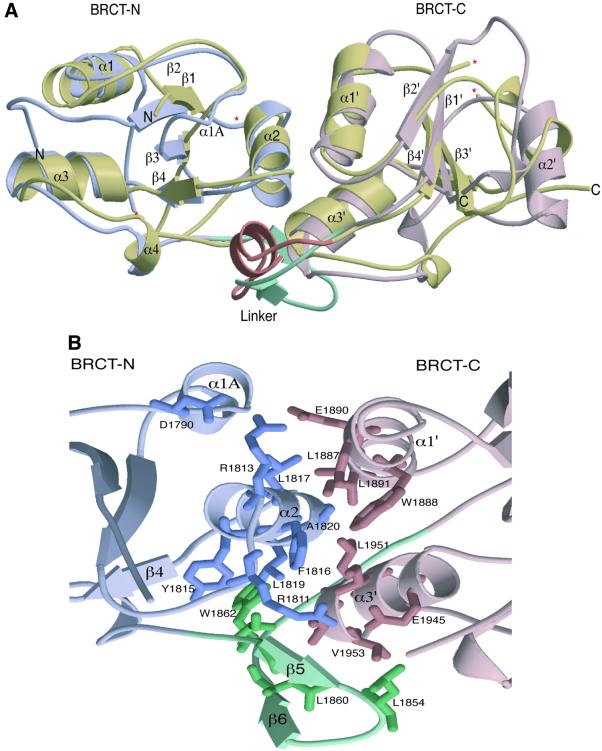
Fig. 2. Structural features of the 53BP1 tandem BRCT repeat. (A) A secondary structure representation of the superimposition of the BRCT tandem repeats from human BRCA1 and 53BP1. The N- and C-terminal BRCT domains of 53BP1 are coloured in light blue and pink, respectively, whilst both BRCT domains of human BRCA1 are coloured gold. The linker regions of each tandem repeat are also highlighted; BRCA1 (a loop–helix–loop structure) in orange and 53BP1 (a β-ribbon-like motif) in green. ‘Missing’ residues in the structure are denoted by an asterisk. (B) Intra-molecular interactions at the inter-BRCT repeat interface of 53BP1. The side chains from the first BRCT repeat are shown in blue, those from the linker are in green and those from the second BRCT repeat are pink. The α2 helix of the first repeat together with α1′ and α3′ helices of the second repeat form a three-helical bundle that is stabilized by α1A and the β-hairpin linker (β5–β6). (C) An amino acid sequence alignment of the regions of BRCA1, 53BP1 and RAD9 that are predicted to form BRCT–BRCT interfaces. Residues that constitute this interface in human 53BP1, as well as conserved residues in human BRCA1 and Sacchromyces cerevisiae RAD9, are coloured red.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.