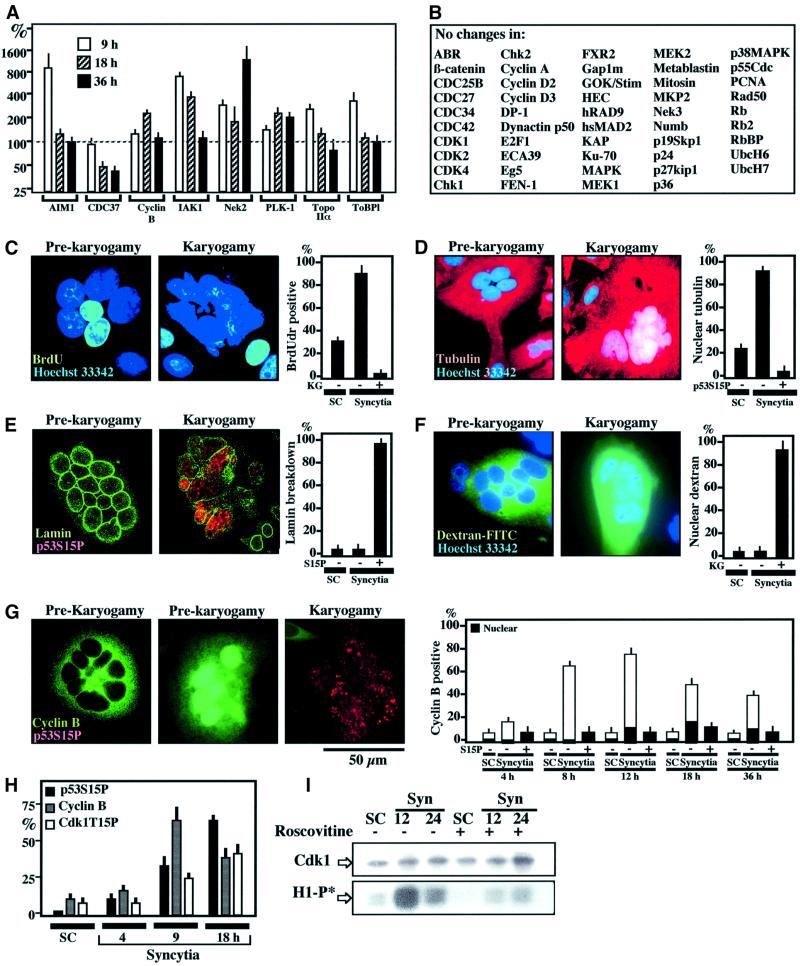
Fig. 2. Phenotypic characterization of Env-induced karyogamy. (A) Cell cycle-relevant proteins whose expression level changes (by a factor of ≥2) upon syncytium formation as determined by quantitative immunoblots (X ± SEM, n = 3). (B) Examples of cell cycle-relevant proteins detectable in HeLa cells whose expression level varies by a factor <2. (C) 5-bromo-2′deoxyuridine (BrdU) incorporation into syncytia as a function of the karyogamy (KG) status. Sixteen-hour-old syncytia were incubated with BrdU for 2 h and then subjected to the immunodetection of BrdU into S-phase nuclei. (D) Tubulin staining of 18-h-old syncytia. Note that the tubulin network is excluded from the nucleus before karyogamy. After karyogamy, nuclei stain positively for tubulin. (E) Lamin B immunostaining of syncytia. Note that karyogamic syncytia exhibit the breakdown of lamin B. (F) Loss of nuclear envelope barrier function in karyogamy. Syncytia were microinjected into the cytoplasm with dextran-70 FITC conjugated, which is excluded from the nucleus of pre-karyogamic cells, yet penetrates into the nucleus of karyogamic syncytia. (G) Cyclin B accumulation in pre-karyogamic syncytia. Two phenotypes (12 h after fusion) are shown, namely pre-karyogamic (p53S15P+) cells exhibiting cyclin B in the cytoplasm or in the nucleus. In contrast, most karyogamic cells lack immunodetectable cyclin B. The kinetics of cyclin B accumulation is shown at different intervals. The few karyogamic (p53S15P+) cells positively staining for cyclin B demonstrate a diffuse (cytoplasmic + nuclear) staining pattern. (H) Kinetics of cyclin B accumulation and the phosphorylation of p53S15 or Cdk1T15. (I) Kinetics of Cdk1 activity. Cdk1 was immunoprecipitated at different intervals after syncytium formation and its capacity to phosphorylate histone H1 in vitro was determined (H1-P*) in the absence or presence of roscovitine (1 µM). Immunoblot detection to confirm equal loading of Cdk1 was also performed.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.