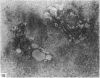
Abstract
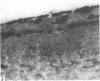
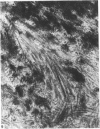
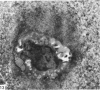
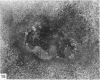
The cationic dye, cupromeronic blue, has been used in a critical electrolyte concentration technique to analyse the ultrastructural changes in cartilage matrix glycosaminoglycans which occur in the dog anterior cruciate ligament division model of osteoarthrosis. Amorphous material appearing at the articular surface of cartilage from the stifle joints of animals subjected to open surgical division of the anterior cruciate ligament has been shown not to comprise glycosaminoglycan. The nature of this material is unknown, but it appears to replace the surface lamina of normal cartilage. It may therefore affect the mechanical properties of the superficial cartilage. The pericellular matrix around single chondrocytes or separating pairs of chondrocytes becomes enriched with sulphated glycosaminoglycan as a response to ligament section. This material is thought to be newly synthesised and secreted and reflects the increased cellular activity resulting from surgically induced canine joint disease.
Full text
PDF

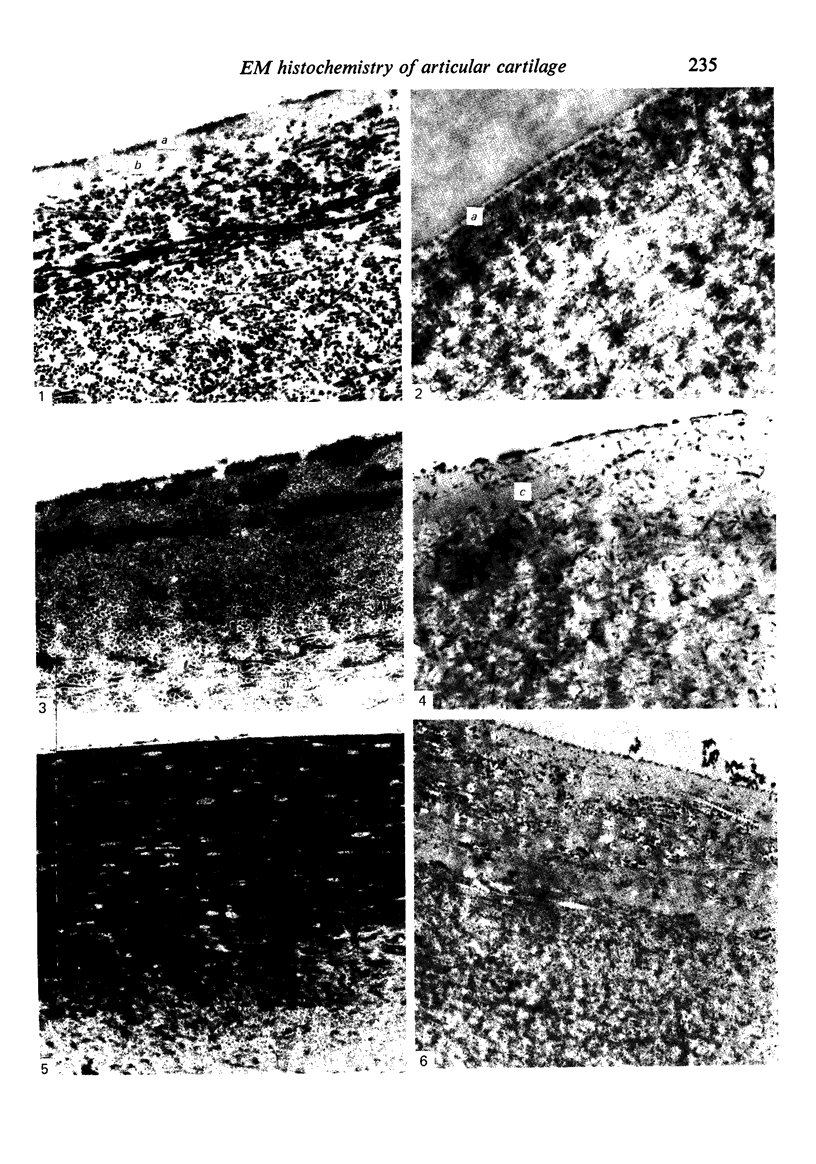

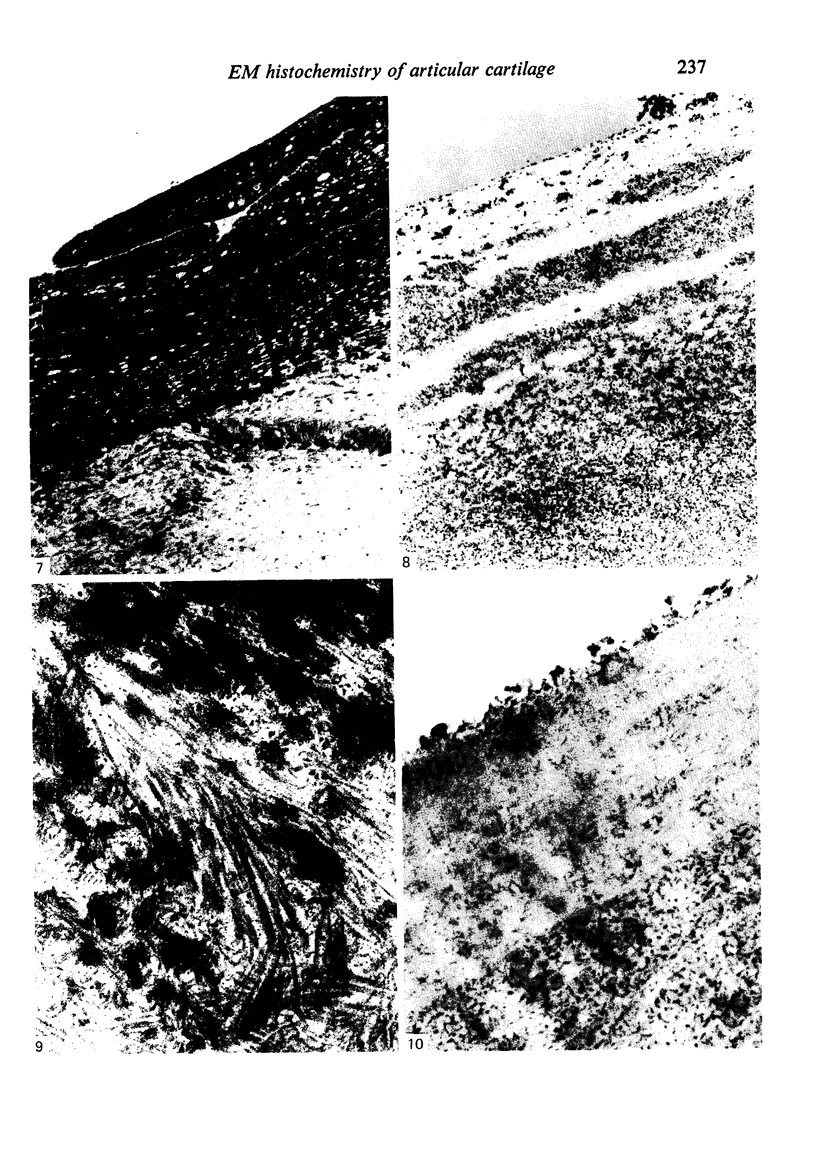

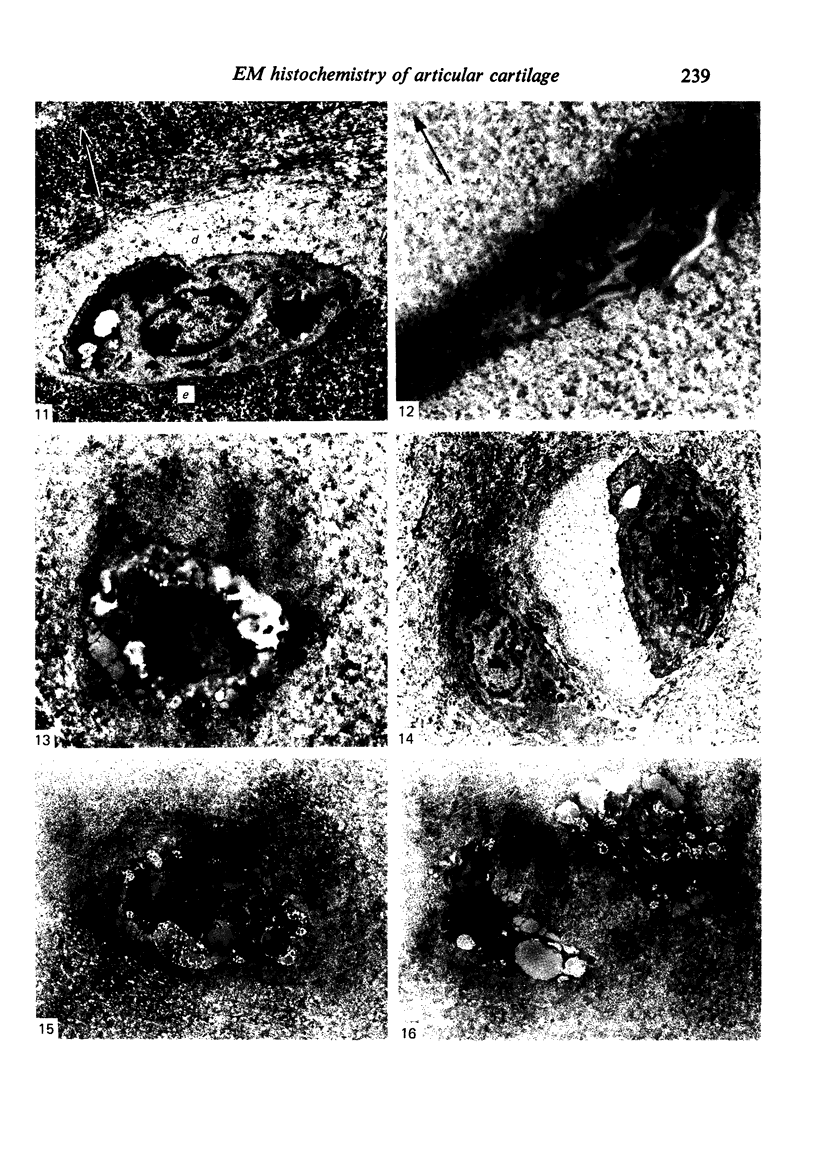





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton-Wurster N., Hui-Chou C. S., Greisen H. A., Lust G. Reduced deposition of collagen in the degenerated articular cartilage of dogs with degenerative joint disease. Biochim Biophys Acta. 1982 Sep 17;718(1):74–84. doi: 10.1016/0304-4165(82)90011-3. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., McDevitt C. A., Billingham M. E., Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980 Jun 15;188(3):823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. L., Bradley W. A., O'Connor P., Orford C. R., Brereton J. D. Synovitis after surgical division of the anterior cruciate ligament of the dog. Clin Exp Rheumatol. 1984 Jan-Mar;2(1):11–15. [PubMed] [Google Scholar]
- Jones I. L., Klämfeldt A., Sandström T. The effect of continuous mechanical pressure upon the turnover of articular cartilage proteoglycans in vitro. Clin Orthop Relat Res. 1982 May;(165):283–289. [PubMed] [Google Scholar]
- Ladefoged C. Amyloid in osteoarthritic hip joints. A pathoanatomical and histological investigation of femoral head cartilage. Acta Orthop Scand. 1982 Aug;53(4):581–586. doi: 10.3109/17453678208992262. [DOI] [PubMed] [Google Scholar]
- Ladefoged C., Christensen H. E. Congophilic substance with green dichroism in hip joints in autopsy material. Acta Pathol Microbiol Scand A. 1980 Jan;88(1):55–58. doi: 10.1111/j.1699-0463.1980.tb02465.x. [DOI] [PubMed] [Google Scholar]
- Lipowitz A. J., Wong P. L., Stevens J. B. Synovial membrane changes after experimental transection of the cranial cruciate ligament in dogs. Am J Vet Res. 1985 May;46(5):1166–1170. [PubMed] [Google Scholar]
- Lippiello L., Hall D., Mankin H. J. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977 Apr;59(4):593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Miller D. R., Lust G. Accumulation of procollagen in the degenerative articular cartilage of dogs with osteoarthritis. Biochim Biophys Acta. 1979 Mar 7;583(2):218–231. doi: 10.1016/0304-4165(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Mitchell N., Shepard N. Pericellular proteoglycan concentrations in early degenerative arthritis. Arthritis Rheum. 1981 Jul;24(7):958–964. doi: 10.1002/art.1780240717. [DOI] [PubMed] [Google Scholar]
- Németh-Csóka M., Mészáros T. Minor collagens in arthrotic human cartilage. Change in content of 1 alpha, 2 alpha, 3 alpha and M-collagen with age and in osteoarthrosis. Acta Orthop Scand. 1983 Aug;54(4):613–619. doi: 10.3109/17453678308992898. [DOI] [PubMed] [Google Scholar]
- O'Connor P., Oates K., Gardner D. L., Middleton J. F., Orford C. R., Brereton J. D. Low temperature and conventional scanning electron microscopic observations of dog femoral condylar cartilage surface after anterior cruciate ligament division. Ann Rheum Dis. 1985 May;44(5):321–327. doi: 10.1136/ard.44.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L., O'Connor P. Ultrastructural changes in dog femoral condylar cartilage following anterior cruciate ligament section. J Anat. 1983 Dec;137(Pt 4):653–663. [PMC free article] [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L. Ultrastructural histochemistry of the surface lamina of normal articular cartilage. Histochem J. 1985 Feb;17(2):223–233. doi: 10.1007/BF01003221. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Effects of static and cyclic compressive loading on articular cartilage plugs in vitro. Arthritis Rheum. 1984 Jun;27(6):675–681. doi: 10.1002/art.1780270611. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Altman R. D., Ghandur-Mnaymneh L., Howell D. S., Woessner J. F., Jr Collagenolytic activity and collagen matrix breakdown of the articular cartilage in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1983 Jul;26(7):866–874. doi: 10.1002/art.1780260708. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Ghandur-Mnaymneh L., Howell D. S., Woessner J. F., Jr Role of synovial membrane inflammation in cartilage matrix breakdown in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1985 May;28(5):554–561. doi: 10.1002/art.1780280515. [DOI] [PubMed] [Google Scholar]
- Poole C. A., Flint M. H., Beaumont B. W. Morphological and functional interrelationships of articular cartilage matrices. J Anat. 1984 Jan;138(Pt 1):113–138. [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Schwartz E. R. Metabolic response during early stages of surgically-induced osteoarthritis in mature beagles. J Rheumatol. 1980 Nov-Dec;7(6):788–800. [PubMed] [Google Scholar]
- Scott J. E. Histochemistry of Alcian blue. 3. The molecular biological basis of staining by Alcian blue 8GX and analogous phthalocyanins. Histochemie. 1972;32(3):191–212. doi: 10.1007/BF00306028. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Billingham M. E., Muir H. Ultrastructural changes in articular cartilage after experimental section of the anterior cruciate ligament of the dog knee. J Anat. 1983 Mar;136(Pt 2):425–439. [PMC free article] [PubMed] [Google Scholar]
- Teshima R., Treadwell B. V., Trahan C. A., Mankin H. J. Comparative rates of proteoglycan synthesis and size of proteoglycans in normal and osteoarthritic chondrocytes. Arthritis Rheum. 1983 Oct;26(10):1225–1230. doi: 10.1002/art.1780261009. [DOI] [PubMed] [Google Scholar]
- Wiltberger H., Lust G. Ultrastructure of canine articular cartilage: comparison of normal and degenerative (osteoarthritic) hip joints. Am J Vet Res. 1975 Jun;36(6):727–740. [PubMed] [Google Scholar]
- Wurster N. B., Lust G. Synthesis of fibronectin in normal and osteoarthritic articular cartilage. Biochim Biophys Acta. 1984 Jul 16;800(1):52–58. doi: 10.1016/0304-4165(84)90093-x. [DOI] [PubMed] [Google Scholar]