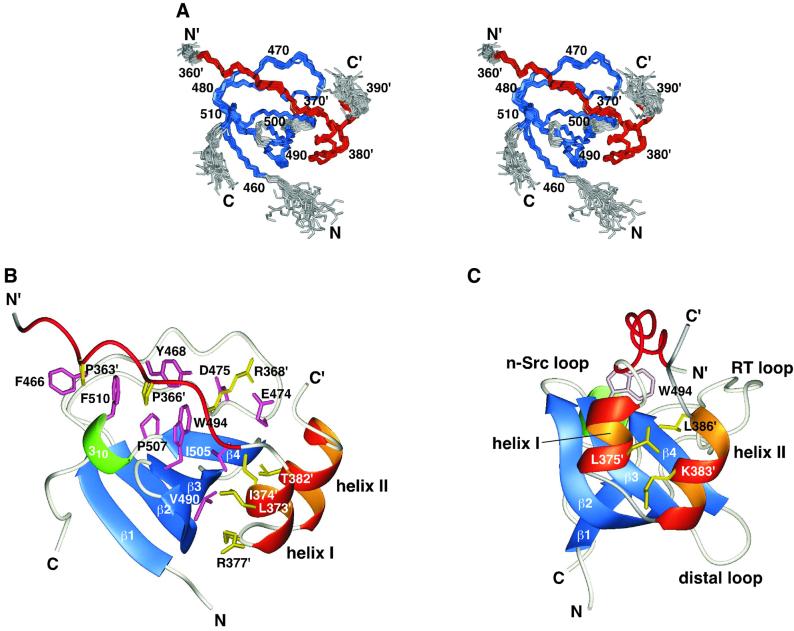
Fig. 5. Structure of the complex of the p67phox SH3(C) and the p47phox tail peptide. (A) Overlay of the 22 NMR structures. The residues used for superimposing the different structures are colored blue (SH3) and red (the tail peptide), and the other residues are in gray. Residue numbers of the p47phox tail peptide are labeled with a prime (′). (B) Ribbon representation of the lowest energy structure. The β-strands of the p67phox SH3(C) are colored blue: β1, residues 460–463; β2, 483–491; β3, 494–498; β4, 503–506; and the 310-helix is colored green: residues 508–510. The PxxP motif and the two α-helices of the p47phox tail peptide are drawn in red and orange, respectively. Side chains located within the binding interface are shown in pink (SH3) and in yellow (the tail peptide). (C) The same structure viewed from a different angle. The side chains of Leu375′, Lys383′ and Leu386′, colored yellow, fill the space between the two α-helices. The positions of the three SH3 loops are shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.