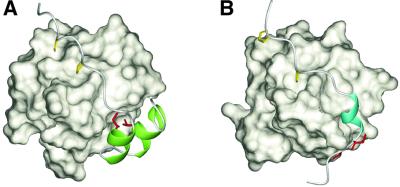
Fig. 6. Comparison of the recognition of the non-PxxP segments outside the conventional PxxP motifs. (A) Ribbon representation of the p47phox tail peptide in complex with the p67phox SH3(C). The residues directly contacting the helix–turn–helix structure (Ile374′ and Thr382′) are colored red, and the two α-helices are in green. The PxxP core proline residues (Pro363′ and Pro366′) are shown in yellow. (B) Ribbon representation of the PEP-3BP1 peptide in complex with the Csk SH3 (PDB entry 1JEG). The hydrophobic residues that are essential for the tight binding (Ile625 and Val626 in full-length PEP) are colored red, and the 310-helix is in cyan. The PxxP core proline residues (Pro614 and Pro617) are shown in yellow.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.