Abstract
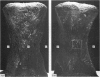
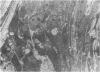
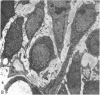
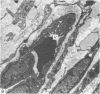
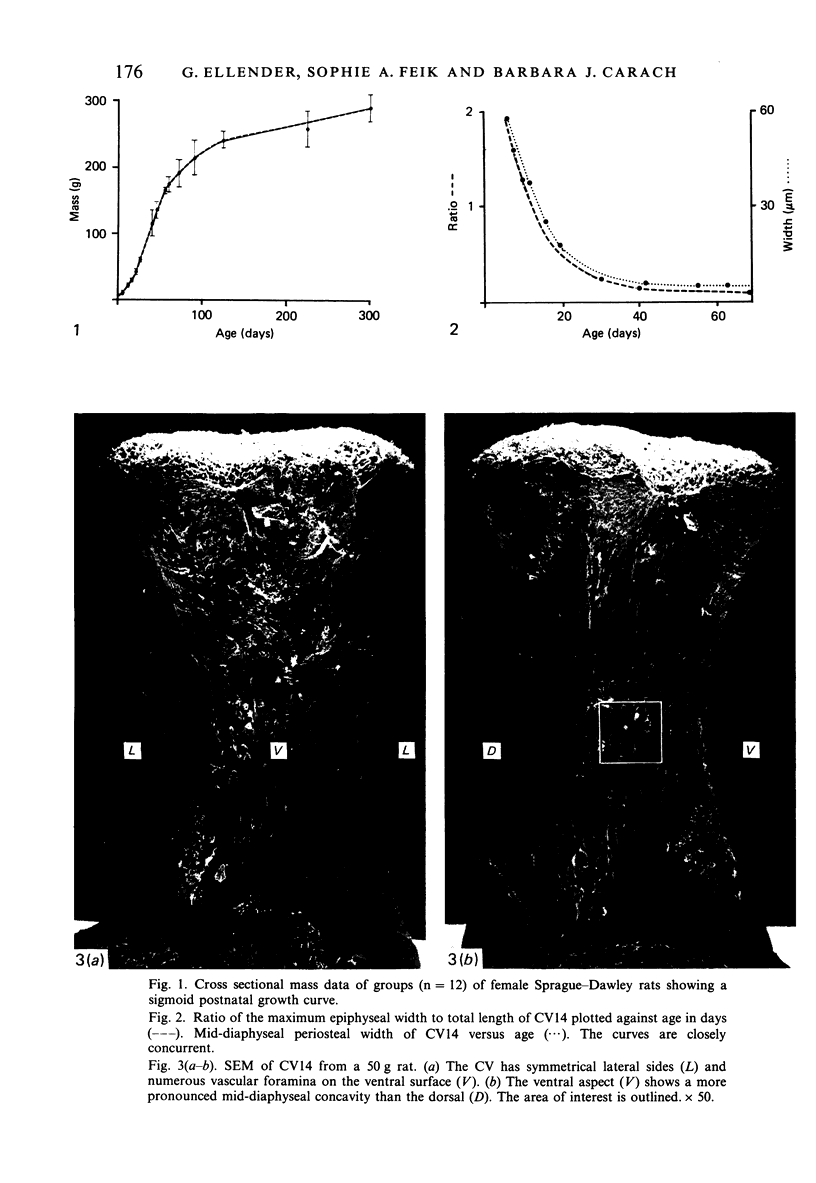
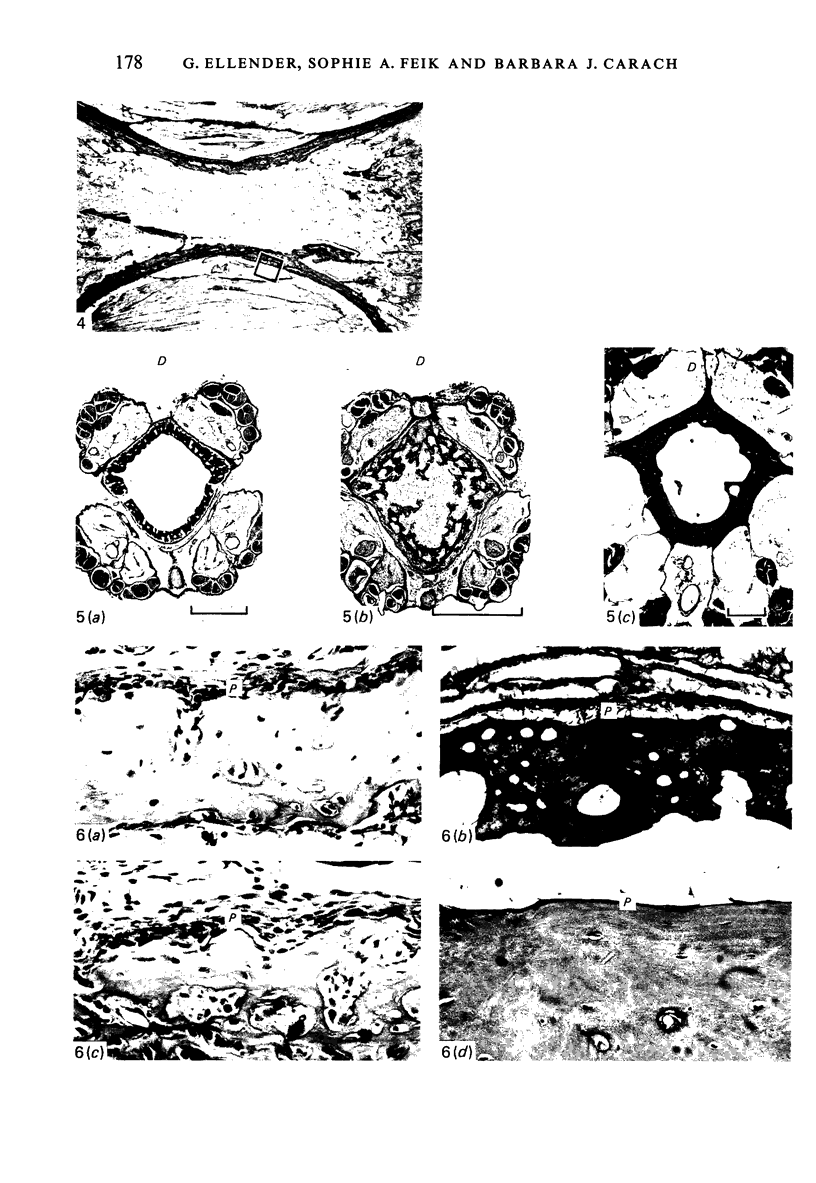
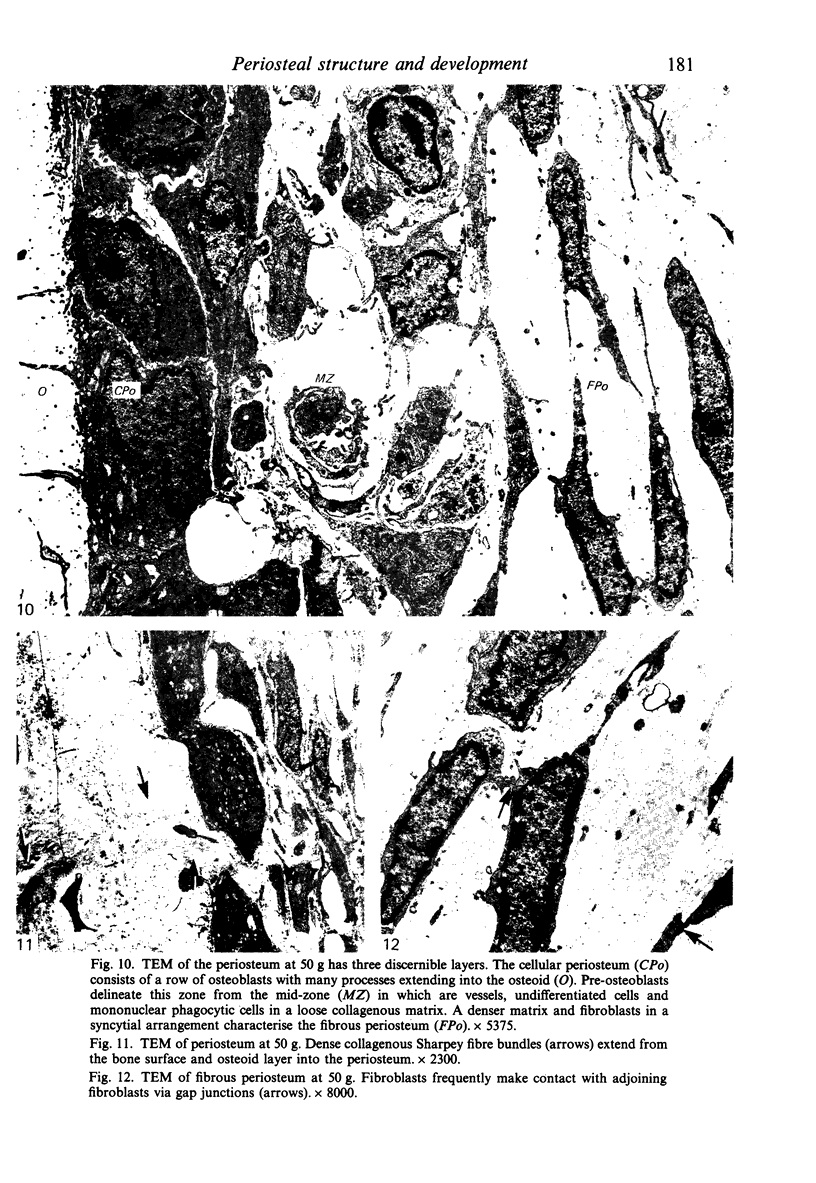
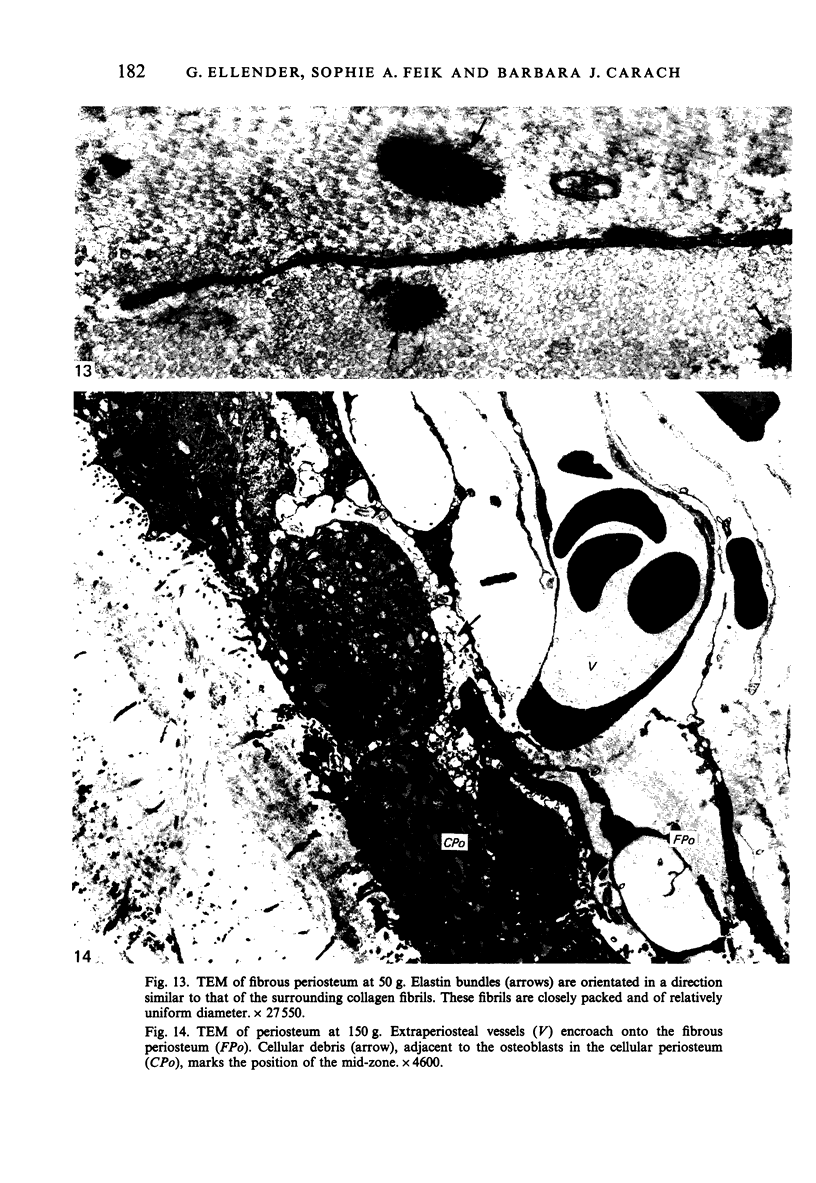
Female Sprague-Dawley rats from birth to 300 days were used to study the bone/soft tissue interrelationships of the 14th caudal vertebra with particular emphasis on the periosteum throughout growth, development and maturation. The growth of the rats follows a sigmoid curve with three phases, a developmental, a rapid growth and a maturation phase. The width/length ratio of the bone and the thickness of the periosteum are closely concurrent, with a rapid decrease during the developmental phase and a levelling off during the rapid growth phase. SEM studies established that the caudal vertebra has symmetrical lateral sides and a pronounced concavity on the ventral surface where the main vascular plexus is located. Morphological changes in the periosteum cna be described as occurring in three layers and reflect the stages seen in general somatic growth. The inner cambial layer initially contains elongated but functional osteoblasts; these become cuboidal during the rapid growth phase and ultimately are flattened and quiescent. The mid-zone with its vessels, undifferentiated and mononuclear phagocytic cells also attains its maximum development in the rapid growth period and then gradually involutes. The fibrous periosteum consists of a syncytial arrangement of fibroblasts in a collagenous matrix which becomes increasingly dense although reduced in width. Sharpey fibre bundles connect the bone with the fibrous periosteum and these become thicker with age. The mid-zone of the periosteum has not been described previously. Besides having a nutritive role and providing progenitor cells it is thought to act as a buffer modulating the interaction between bone and the covering soft tissues. With age and the deletion of the mid-zone a less sensitive periosteal response to stress can be expected.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHESON R. M., MACINTYRE M. N., OLDHAM E. Techniques in longitudinal studies of the skeletal development of the rat. Br J Nutr. 1959;13:283–292. doi: 10.1079/bjn19590039. [DOI] [PubMed] [Google Scholar]
- Altman J., Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975 Nov;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Ashhurst D. E. The influence of mechanical conditions on the healing of experimental fractures in the rabbit: a microscopical study. Philos Trans R Soc Lond B Biol Sci. 1986 Sep 24;313(1161):271–302. doi: 10.1098/rstb.1986.0038. [DOI] [PubMed] [Google Scholar]
- Baird I. L., Winborn W. B., Bockman D. E. A technique of decalcification suited to electron microscopy of tissues closely associated with bone. Anat Rec. 1967 Nov;159(3):281–289. doi: 10.1002/ar.1091590306. [DOI] [PubMed] [Google Scholar]
- Beaulaton J., Lockshin R. A. The relation of programmed cell death to development and reproduction: comparative studies and an attempt at classification. Int Rev Cytol. 1982;79:215–235. doi: 10.1016/s0074-7696(08)61675-7. [DOI] [PubMed] [Google Scholar]
- Chong D. A., Evans C. A., Heeley J. D. Morphology and maturation of the periosteum of the rat mandible. Arch Oral Biol. 1982;27(9):777–785. doi: 10.1016/0003-9969(82)90029-2. [DOI] [PubMed] [Google Scholar]
- Chong D. A., Evans C. A. Histologic study of the attachment of muscles to the rat mandible. Arch Oral Biol. 1982;27(7):519–527. doi: 10.1016/0003-9969(82)90065-6. [DOI] [PubMed] [Google Scholar]
- Cleary E. G., Gibson M. A. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res. 1983;10:97–209. doi: 10.1016/b978-0-12-363710-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Crilly R. G. Longitudinal overgrowth of chicken radius. J Anat. 1972 May;112(Pt 1):11–18. [PMC free article] [PubMed] [Google Scholar]
- Feik S. A., Storey E. Remodelling of bone and bones: growth of normal and transplanted caudal vertebrae. J Anat. 1983 Jan;136(Pt 1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Harkness E. M., Trotter W. D. Growth of transplants of rat humerus following circumferential division of the periosteum. J Anat. 1978 Jun;126(Pt 2):275–289. [PMC free article] [PubMed] [Google Scholar]
- Junqueira L. C., Bignolas G., Brentani R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Lutfi A. M. The role of cartilage in long bone growth: a reappraisal. J Anat. 1974 Apr;117(Pt 2):413–417. [PMC free article] [PubMed] [Google Scholar]
- Ord M. J., Smith R. A. Correlation of mitochondrial form and function in vivo: microinjection of substrate and nucleotides. Cell Tissue Res. 1982;227(1):129–137. doi: 10.1007/BF00206336. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Gilula N. B. Gap junctions in normal and transformed fibroblasts in culture. Exp Cell Res. 1972;71(2):393–401. doi: 10.1016/0014-4827(72)90309-6. [DOI] [PubMed] [Google Scholar]
- Pollard A. W., Feik S. A., Storey E. Remodelling of bone and bones: effects of translation and strain on transplants. Br J Exp Pathol. 1984 Dec;65(6):655–670. [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Storey E., Feik S. A. Remodeling of bone and bones: effects of altered mechanical stress on the regeneration of transplanted bones. Anat Rec. 1986 Jun;215(2):153–166. doi: 10.1002/ar.1092150209. [DOI] [PubMed] [Google Scholar]
- Storey E., Feik S. A. Remodelling of bone and bones. Effects of altered mechanical stress on anlages. Br J Exp Pathol. 1982 Apr;63(2):184–193. [PMC free article] [PubMed] [Google Scholar]
- Storey E., Feik S. A. Remodelling of bone and bones: effects of altered mechanical stress on caudal vertebrae. J Anat. 1985 Jan;140(Pt 1):37–48. [PMC free article] [PubMed] [Google Scholar]
- Tonna E. A. Electron microscopy of aging skeletal cells. III. The periosteum. Lab Invest. 1974 Dec;31(6):609–632. [PubMed] [Google Scholar]
- Tonna E. A. Response of the cellular phase of the skeleton to trauma. Periodontics. 1966 May-Jun;4(3):105–114. [PubMed] [Google Scholar]
- Warrell E., Taylor J. F. The role of periosteal tension in the growth of long bones. J Anat. 1979 Jan;128(Pt 1):179–184. [PMC free article] [PubMed] [Google Scholar]
- Watters W. B., Buck R. C. An improved simple method of specimen preparation for replicas or scanning electron microscopy. J Microsc. 1971 Oct;94(2):185–187. doi: 10.1111/j.1365-2818.1971.tb03703.x. [DOI] [PubMed] [Google Scholar]