Abstract
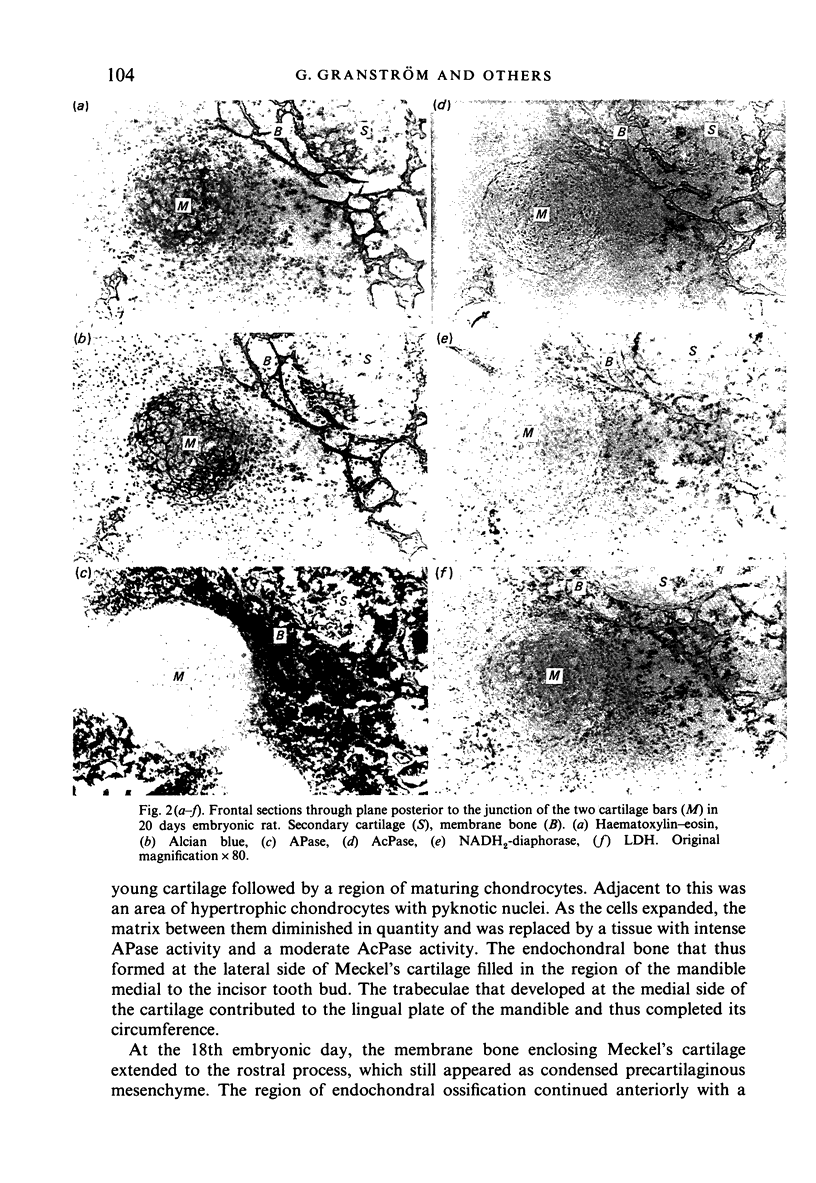
Osteogenesis of the body of the mandible in embryonic and neonatal rats was studied histologically and by histochemistry to determine the role of Meckel's cartilage in bone formation. Meckel's cartilage showed intense activity of lactate dehydrogenase and NADH2-diaphorase and weak activity of acid phosphatase, indicating a functioning citric acid cycle, pentose phosphate shunt and a capacity for anaerobic metabolism. The activity of these enzymes declined after hypertrophy of Meckel's cartilage. Alkaline phosphatase was the major enzyme of mineralising mandibular osteoid and was present in the osteoblasts and osteoprogenitor cells but not in Meckel's cartilage. After the differentiation of Meckel's cartilage and intramembranous bone, Meckel's cartilage supported mandibular bone formation by endochondral ossification in the anterior part of the mandible.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akisaka T. The localization of thiamine pyrophosphatase activity in Meckel's cartilage cells during endochondral ossification. Histochemistry. 1982;76(4):539–546. doi: 10.1007/BF00489908. [DOI] [PubMed] [Google Scholar]
- BHASKAR S. N. Growth pattern of the rat mandible from 13 days insemination age to 30 days after birth. Am J Anat. 1953 Jan;92(1):1–53. doi: 10.1002/aja.1000920102. [DOI] [PubMed] [Google Scholar]
- BHASKAR S. N., WEINMANN J. P., SCHOUR I. Role of Meckel's cartilage in the development and growth of the rat mandible. J Dent Res. 1953 Jun;32(3):398–410. doi: 10.1177/00220345530320031401. [DOI] [PubMed] [Google Scholar]
- BURSTONE M. S. Histochemical observations on enzymatic processes in bones and teeth. Ann N Y Acad Sci. 1960 Mar 29;85:431–444. doi: 10.1111/j.1749-6632.1960.tb49972.x. [DOI] [PubMed] [Google Scholar]
- Baylink D., Wergedal J., Thompson E. Loss of proteinpolysaccharides at sites where bone mineralization is initiated. J Histochem Cytochem. 1972 Apr;20(4):279–292. doi: 10.1177/20.4.279. [DOI] [PubMed] [Google Scholar]
- Bee J., Thorogood P. The role of tissue interactions in the skeletogenic differentiation of avian neural crest cells. Dev Biol. 1980 Jul;78(1):47–62. doi: 10.1016/0012-1606(80)90317-6. [DOI] [PubMed] [Google Scholar]
- FLEISH H., NEUMAN W. F. Mechanisms of calcification: role of collagen, polyphosphates, and phosphatase. Am J Physiol. 1961 Jun;200:1296–1300. doi: 10.1152/ajplegacy.1961.200.6.1296. [DOI] [PubMed] [Google Scholar]
- Frommer J., Margolies M. R. Contribution of Meckel's cartilage to ossification of the mandible in mice. J Dent Res. 1971 Sep-Oct;50(5):1260–1267. doi: 10.1177/00220345710500052801. [DOI] [PubMed] [Google Scholar]
- Granström G., Magnusson B. C. Changes in alkaline-phosphatase isoenzymes of hard tissue origin during facial development in the rat. Arch Oral Biol. 1986;31(8):513–519. doi: 10.1016/0003-9969(86)90144-5. [DOI] [PubMed] [Google Scholar]
- Granström G., Magnusson B. C. Lactate dehydrogenase isoenzyme changes during facial development. J Anat. 1986 Oct;148:183–192. [PMC free article] [PubMed] [Google Scholar]
- Hall B. K., Tremaine R. Ability of neural crest cells from the embryonic chick to differentiate into cartilage before their migration away from the neural tube. Anat Rec. 1979 Jul;194(3):469–475. doi: 10.1002/ar.1091940312. [DOI] [PubMed] [Google Scholar]
- Heyden G., From S. H. A histochemical study of some oxidative enzymes in mouse molar ontogeny. Arch Oral Biol. 1969 Oct;14(10):1233–1241. doi: 10.1016/0003-9969(69)90161-7. [DOI] [PubMed] [Google Scholar]
- Heyden G., From S. H. Enzyme histochemistry and its application in comparative studies of adenosinetriphosphatase (ATPase) and some oxidative enzymes in bone, cartilage and tooth germs. Odontol Revy. 1970;21(2):129–142. [PubMed] [Google Scholar]
- Kjaer I. Histochemical investigations on the symphysis menti in the human fetus related to fetal skeletal maturation in the hand and foot. Acta Anat (Basel) 1975;93(4):606–633. [PubMed] [Google Scholar]
- Nilsen R., Magnusson B. C. Enzyme histochemistry of induced heterotropic bone formation in guinea-pigs. Arch Oral Biol. 1979;24(10-11):833–841. doi: 10.1016/0003-9969(79)90047-5. [DOI] [PubMed] [Google Scholar]
- RICHANY S. F., BAST T. H., ANSON B. J. The development of the first branchial arch in man and the fate of Meckel's cartilage. Q Bull Northwest Univ Med Sch. 1956;30(4):331–355. [PMC free article] [PubMed] [Google Scholar]
- Thorogood P. V., Hall B. K. The use of variable lactate/malic dehydrogenase ratios to distinguish between progenitor cells of cartilage and bone in the embryonic chick. J Embryol Exp Morphol. 1976 Oct;36(2):305–313. [PubMed] [Google Scholar]
- Tyler M. S., Hall B. K. Epithelial influences on skeletogenesis in the mandible of the embryonic chick. Anat Rec. 1977 Jun;188(2):229–239. doi: 10.1002/ar.1091880208. [DOI] [PubMed] [Google Scholar]