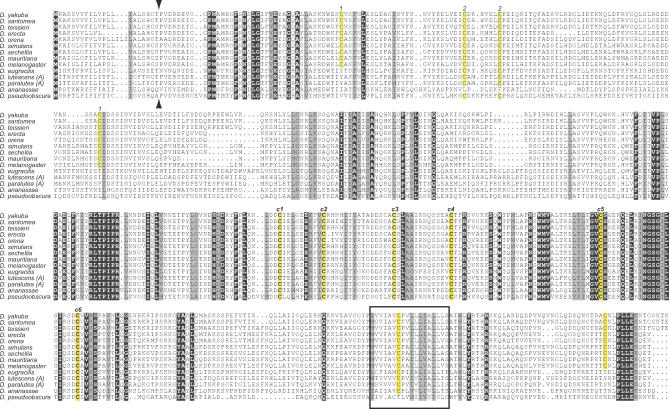
Figure 5. Complete Alignment of Iris Proteins.
An alignment of full-length Iris proteins from various Drosophila species is shown. All invariant residues are shown against a black background (except cysteines that are highlighted in yellow), while similar residues are highlighted in gray background. We did not include the Iris-B lineage here for ease of presentation (these are presented in Figure 6). Several features are conserved, including the signal peptide (predicted cleavage site indicated by arrowheads), C-terminal transmembrane domain (shown as a box), and several invariant cysteine residues (c1 through c6, highlighted in yellow) that are a characteristic feature of Iris and related envelope proteins. Other cysteine residue pairs (1–1 and 2–2, also highlighted in yellow) show co-conservation, i.e., loss of one results in loss of the other.