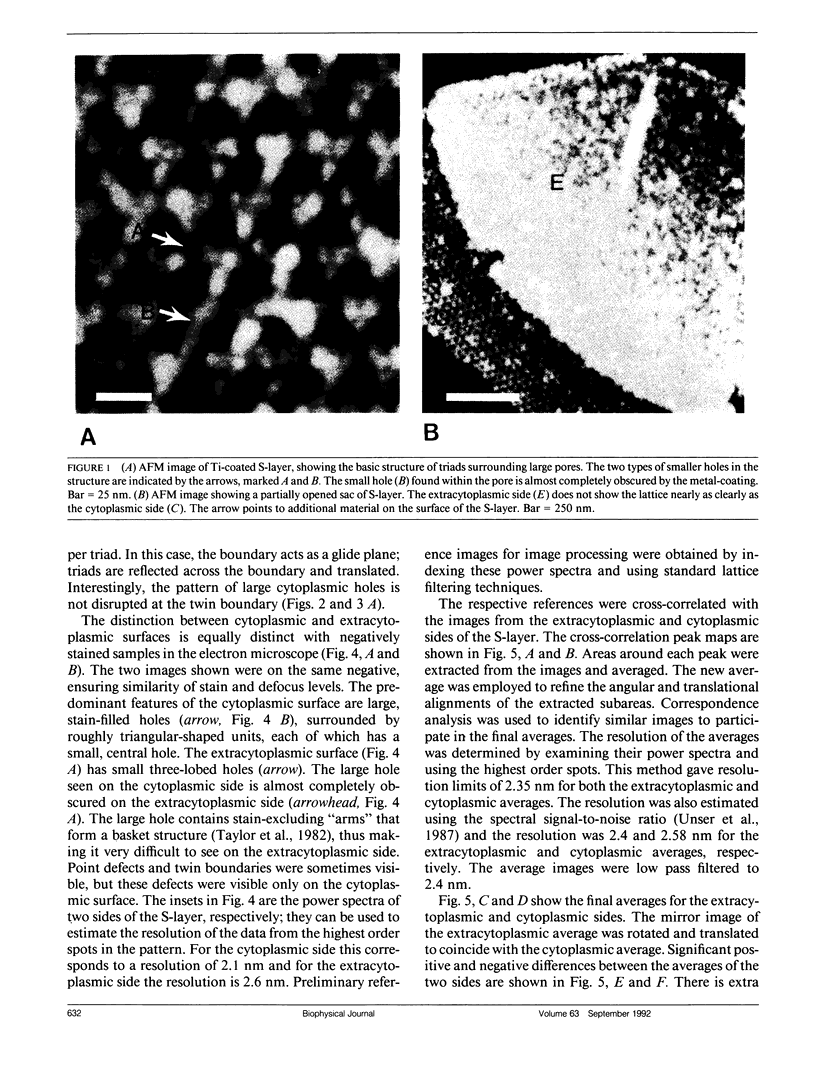
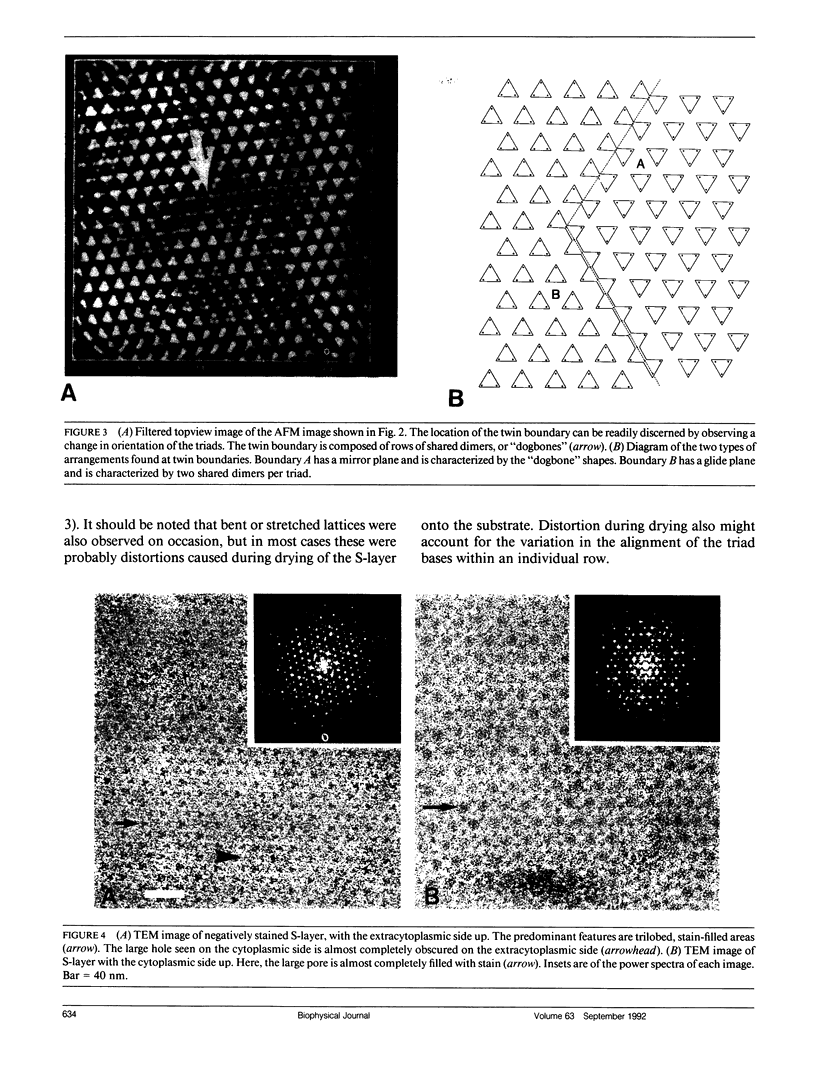
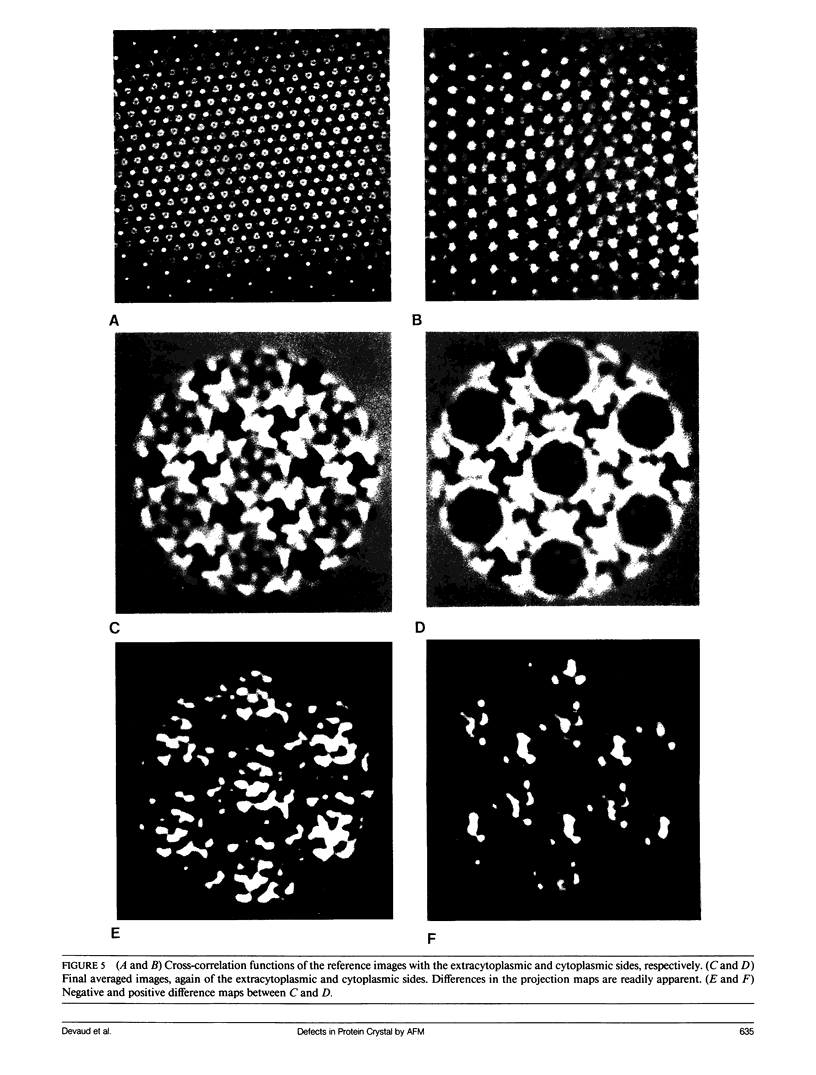
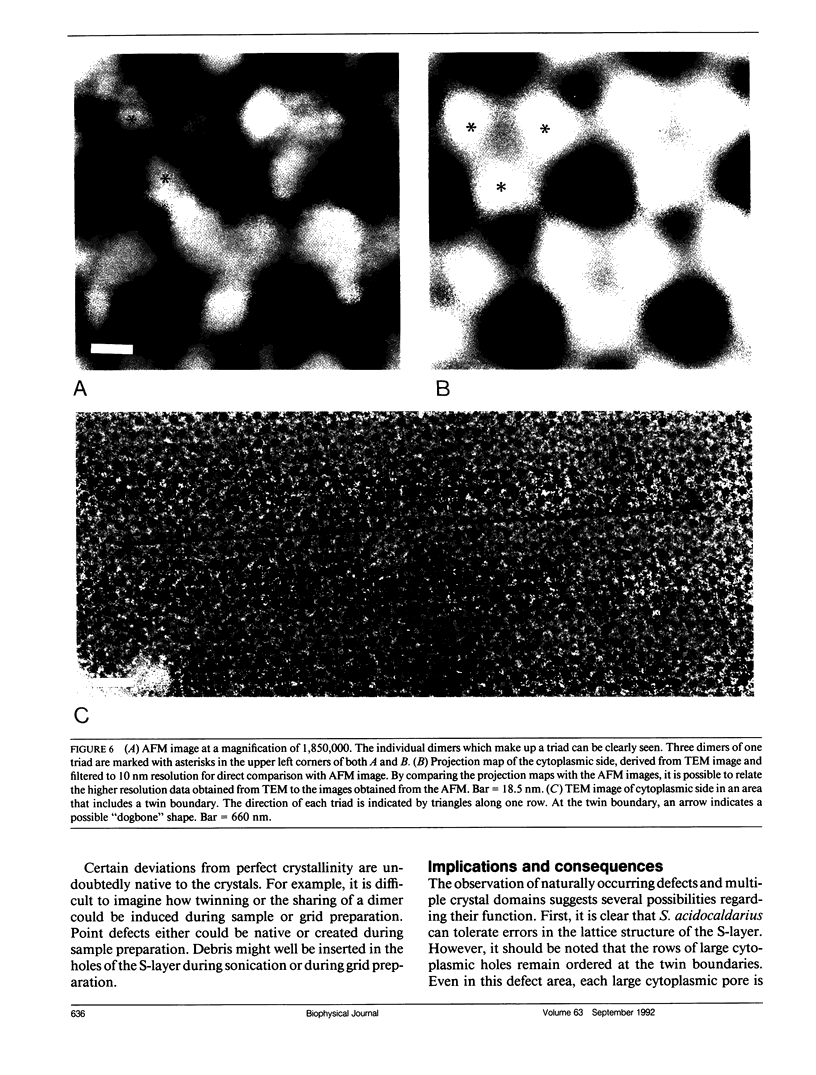
Abstract
We have examined the structure of S-layers isolated from Sulfolobus acidocaldarius using atomic force microscopy (AFM) and transmission electron microscopy (TEM). From the AFM images, we were able to directly observe individual dimers of the crystal, defects in the crystal structure, and twin boundaries. We have identified two types of boundaries, one defined by a mirror plane and the other by a glide plane. This work shows that twin boundaries are highly structured regions that are directly related to the organization of units within each crystal domain. Projection maps from TEM images have shown that there are significant differences in the final average maps has allowed us to relate high magnification views obtained by AFM to the relatively high resolution information obtained by electron microscopy and image processing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Bretaudiere J. P., Frank J. Reconstitution of molecule images analysed by correspondence analysis: a tool for structural interpretation. J Microsc. 1986 Oct;144(Pt 1):1–14. doi: 10.1111/j.1365-2818.1986.tb04669.x. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Furcinitti P. S., van Oostrum J., Burnett R. M. Adenovirus polypeptide IX revealed as capsid cement by difference images from electron microscopy and crystallography. EMBO J. 1989 Dec 1;8(12):3563–3570. doi: 10.1002/j.1460-2075.1989.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma P. K., Elings V. B., Marti O., Bracker C. E. Scanning tunneling microscopy and atomic force microscopy: application to biology and technology. Science. 1988 Oct 14;242(4876):209–216. doi: 10.1126/science.3051380. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Whittaker M., Safer D. Molecular structure of F-actin and location of surface binding sites. Nature. 1990 Nov 15;348(6298):217–221. doi: 10.1038/348217a0. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Unser M., Trus B. L., Steven A. C. A new resolution criterion based on spectral signal-to-noise ratios. Ultramicroscopy. 1987;23(1):39–51. doi: 10.1016/0304-3991(87)90225-7. [DOI] [PubMed] [Google Scholar]








