Abstract
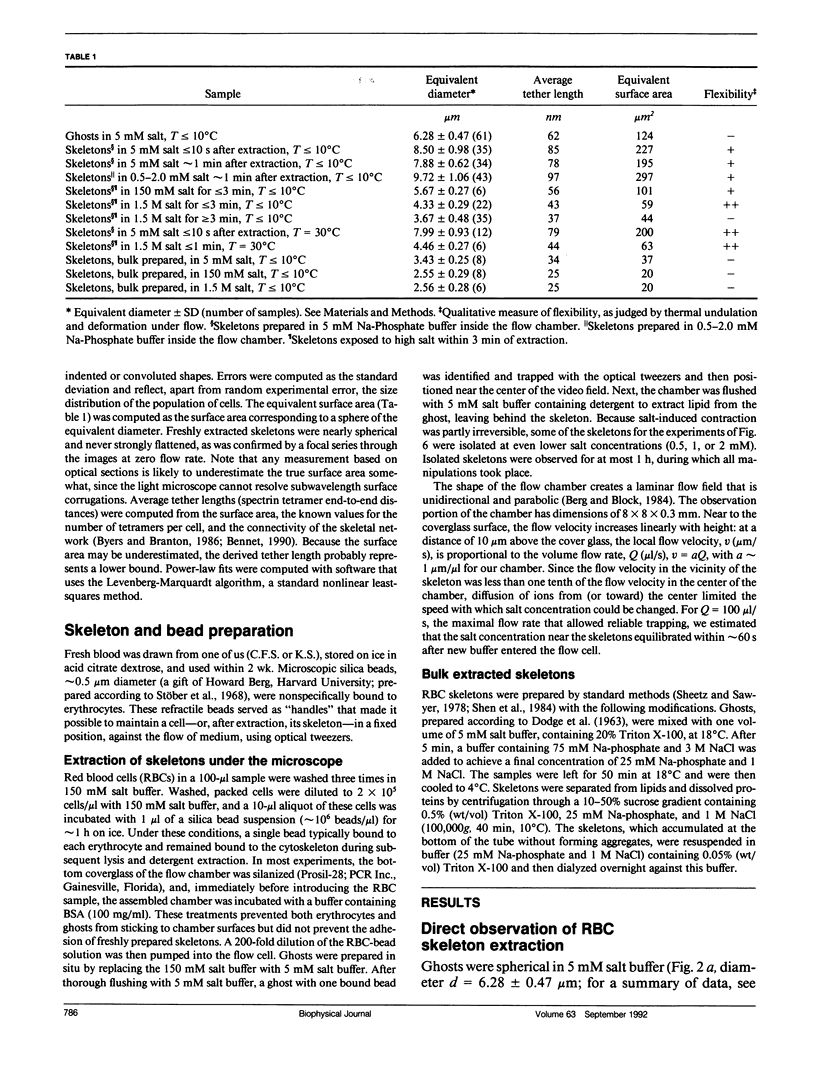
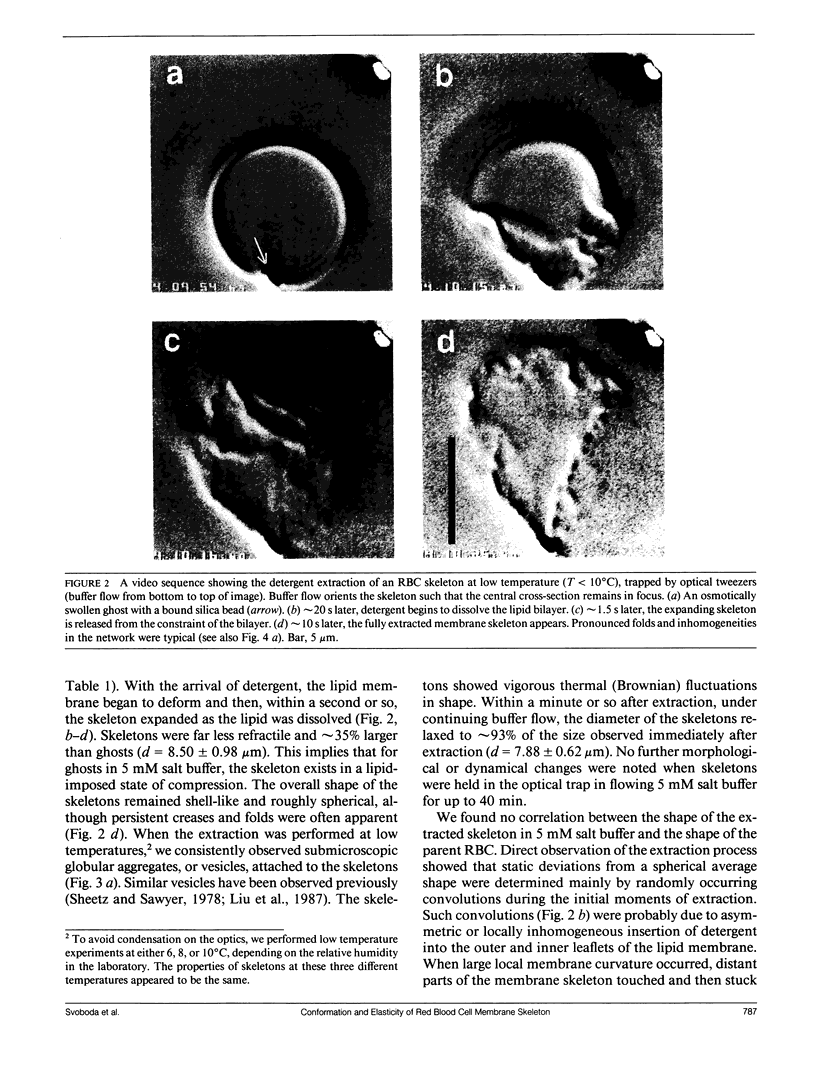
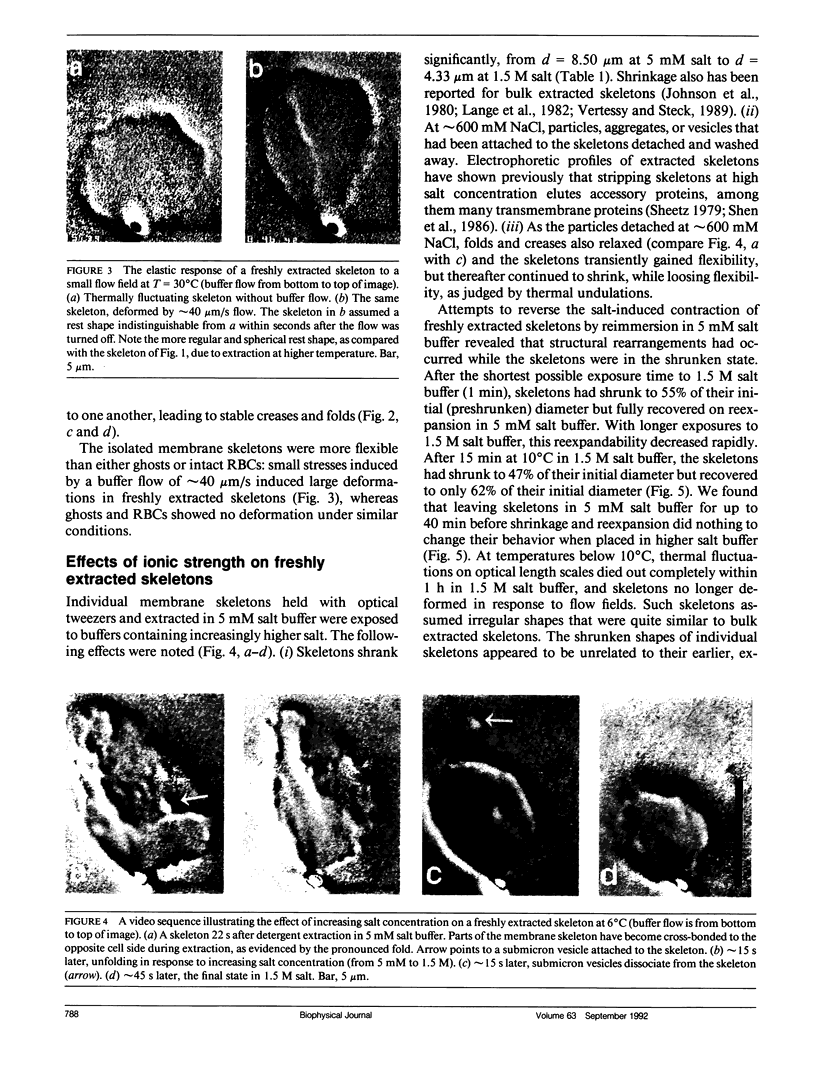
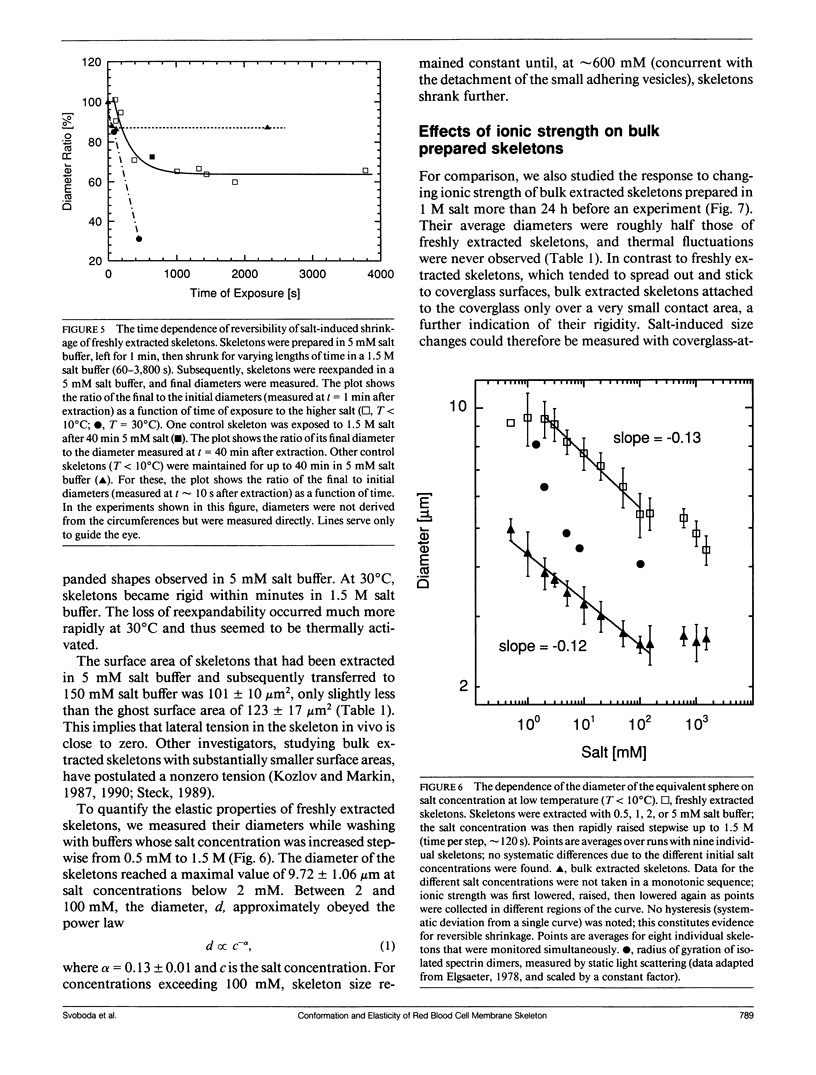
We studied the structure and elasticity of membrane skeletons from human red blood cells (RBCs) during and after extraction of RBC ghosts with nonionic detergent. Optical tweezers were used to suspend individual cells inside a flow chamber, away from all surfaces; this procedure allowed complete exchange of medium while the low-contrast protein network of the skeleton was observed by high resolution, video-enhanced differential interference-contrast (DIC) microscopy. Immediately following extraction in a 5 mM salt buffer, skeletons assumed expanded, nearly spherical shapes that were uncorrelated with the shapes of their parent RBCs. Judging by the extent of thermal undulations and by their deformability in small flow fields, the bending rigidity of skeletons was markedly lower than that of either RBCs or ghosts. No further changes were apparent in skeletons maintained in this buffer for up to 40 min at low temperatures (T less than 10 degrees C), but skeletons shrank when the ionic strength of the buffer was increased. When the salt concentration was raised to 1.5 M, shrinkage remained reversible for approximately 1 min but thereafter became irreversible. When maintained in 1.5 M salt buffer for longer periods, skeletons continued to shrink, lost flexibility, and assumed irregular shapes: this rigidification was irreversible. At this stage, skeletons closely resembled those isolated in standard bulk preparations. We propose that the transformation to the rigid, irreversibly shrunken state is a consequence of spectrin dimer-dimer reconnections and that these structural rearrangements are thermally activated. We also measured the salt-dependent size of fresh and bulk extracted skeletons. Our measurements suggest that, in situ, the spectrin tethers are flexible, with a persistence length of approximately 10 nm at 150 mM salt.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronovitz JA, Lubensky TC. Fluctuations of solid membranes. Phys Rev Lett. 1988 Jun 20;60(25):2634–2637. doi: 10.1103/PhysRevLett.60.2634. [DOI] [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Block S. M. A miniature flow cell designed for rapid exchange of media under high-power microscope objectives. J Gen Microbiol. 1984 Nov;130(11):2915–2920. doi: 10.1099/00221287-130-11-2915. [DOI] [PubMed] [Google Scholar]
- Block S. M., Fahrner K. A., Berg H. C. Visualization of bacterial flagella by video-enhanced light microscopy. J Bacteriol. 1991 Jan;173(2):933–936. doi: 10.1128/jb.173.2.933-936.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull B. S., Weinstein R. S., Korpman R. A. On the thickness of the red cell membrane skeleton: quantitative electron microscopy of maximally narrowed isthmus regions of intact cells. Blood Cells. 1986;12(1):25–42. [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A. Human spectrin. I. A classical light scattering study. Biochim Biophys Acta. 1978 Sep 26;536(1):235–244. doi: 10.1016/0005-2795(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Stokke B. T., Mikkelsen A., Branton D. The molecular basis of erythrocyte shape. Science. 1986 Dec 5;234(4781):1217–1223. doi: 10.1126/science.3775380. [DOI] [PubMed] [Google Scholar]
- Evans E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 1973 Sep;13(9):941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M., Haest C. W., Stöhr-Liesen M., Schmid-Schönbein H., Skalak R. The stress-free shape of the red blood cell membrane. Biophys J. 1981 Jun;34(3):409–422. doi: 10.1016/S0006-3495(81)84859-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. M. Role of spectrin in cross bonding of the red cell membrane. Blood Cells. 1988;13(3):377–396. [PubMed] [Google Scholar]
- Johnson R. M., Taylor G., Meyer D. B. Shape and volume changes in erythrocyte ghosts and spectrin-actin networks. J Cell Biol. 1980 Aug;86(2):371–376. doi: 10.1083/jcb.86.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. M., Markin V. S. Model of red blood cell membrane skeleton: electrical and mechanical properties. J Theor Biol. 1987 Dec 21;129(4):439–452. doi: 10.1016/s0022-5193(87)80023-1. [DOI] [PubMed] [Google Scholar]
- Lange Y., Hadesman R. A., Steck T. L. Role of the reticulum in the stability and shape of the isolated human erythrocyte membrane. J Cell Biol. 1982 Mar;92(3):714–721. doi: 10.1083/jcb.92.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol. 1987 Mar;104(3):527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Derick L. H., Zhai S., Palek J. Uncoupling of the spectrin-based skeleton from the lipid bilayer in sickled red cells. Science. 1991 Apr 26;252(5005):574–576. doi: 10.1126/science.2020854. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A. M., Josephs R. On the structure of erythrocyte spectrin in partially expanded membrane skeletons. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5208–5212. doi: 10.1073/pnas.87.13.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta. 1979 Oct 19;557(1):122–134. doi: 10.1016/0005-2736(79)90095-6. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Sawyer D. Triton shells of intact erythrocytes. J Supramol Struct. 1978;8(4):399–412. doi: 10.1002/jss.400080403. [DOI] [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of the intact skeleton of the human erythrocyte membrane. J Cell Biol. 1986 Mar;102(3):997–1006. doi: 10.1083/jcb.102.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. W., Josephs R., Steck T. L. Ultrastructure of unit fragments of the skeleton of the human erythrocyte membrane. J Cell Biol. 1984 Sep;99(3):810–821. doi: 10.1083/jcb.99.3.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Stokke B. T., Mikkelsen A., Elgsaeter A. Human erythrocyte spectrin dimer intrinsic viscosity: temperature dependence and implications for the molecular basis of the erythrocyte membrane free energy. Biochim Biophys Acta. 1985 Jun 11;816(1):102–110. doi: 10.1016/0005-2736(85)90398-0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Gratzer W. Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem. 1978 Aug 1;88(2):379–385. doi: 10.1111/j.1432-1033.1978.tb12459.x. [DOI] [PubMed] [Google Scholar]
- Vertessy B. G., Steck T. L. Elasticity of the human red cell membrane skeleton. Effects of temperature and denaturants. Biophys J. 1989 Feb;55(2):255–262. doi: 10.1016/S0006-3495(89)82800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E., Agre P. Reductions of erythrocyte membrane viscoelastic coefficients reflect spectrin deficiencies in hereditary spherocytosis. J Clin Invest. 1988 Jan;81(1):133–141. doi: 10.1172/JCI113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R. E. Effects of inherited membrane abnormalities on the viscoelastic properties of erythrocyte membrane. Biophys J. 1987 Mar;51(3):363–369. doi: 10.1016/S0006-3495(87)83358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Evans E. A. Thermoelasticity of red blood cell membrane. Biophys J. 1979 Apr;26(1):115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Fischman D. A., Steck T. L. Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct. 1973;1(3):233–248. doi: 10.1002/jss.400010308. [DOI] [PubMed] [Google Scholar]