Abstract
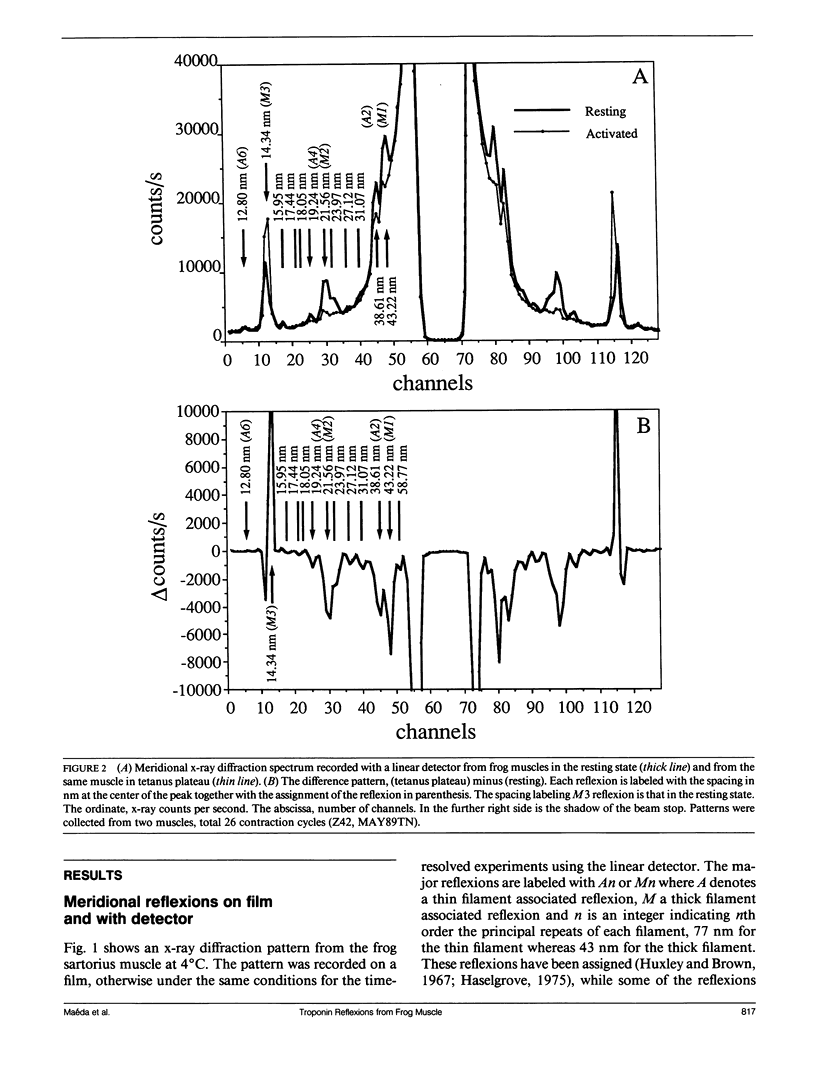
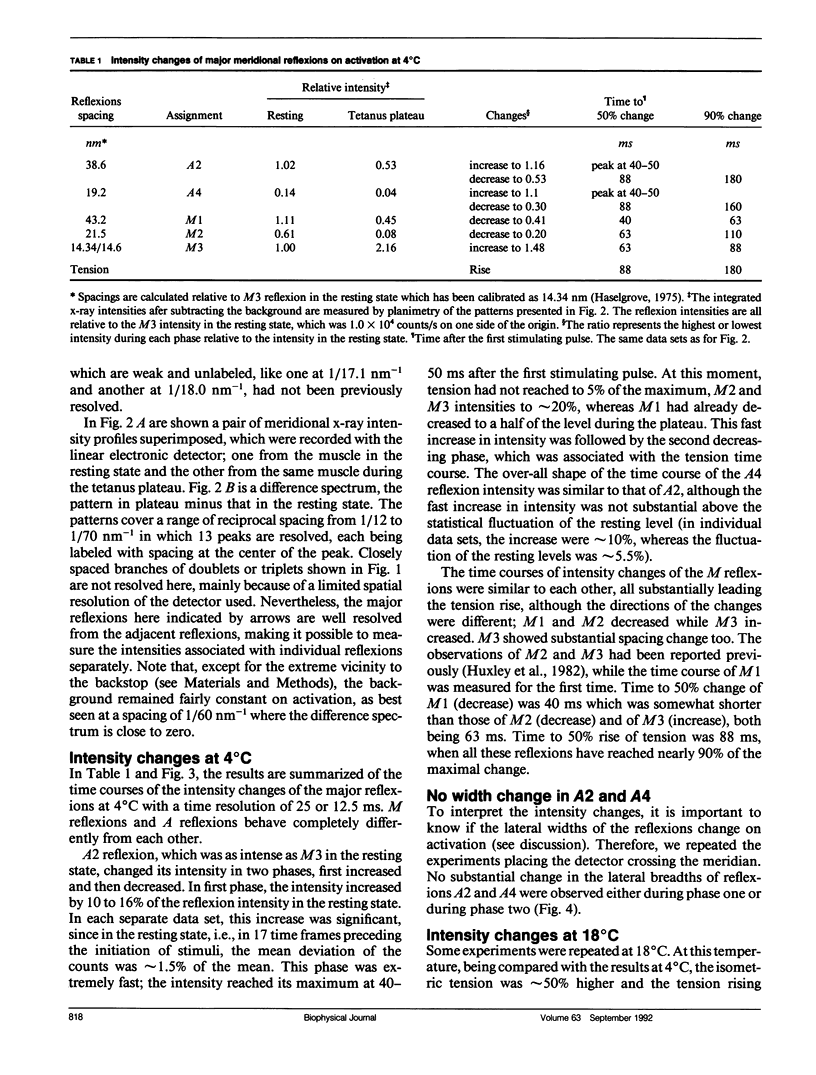
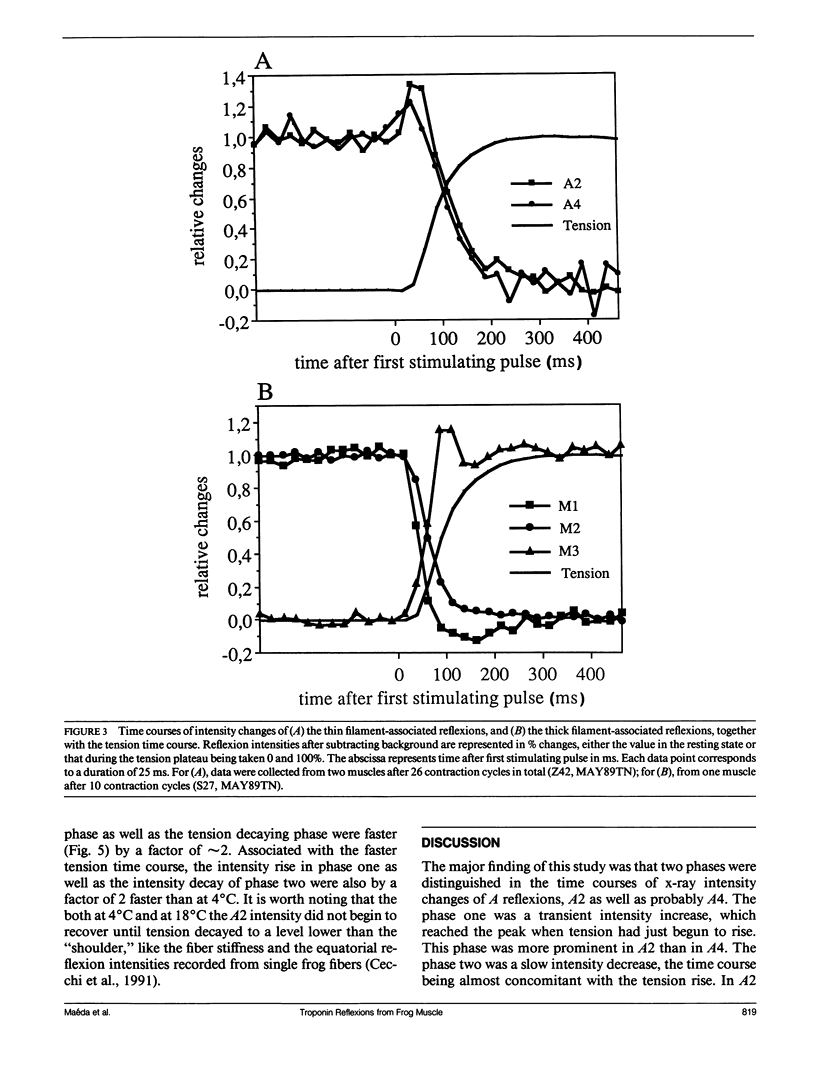
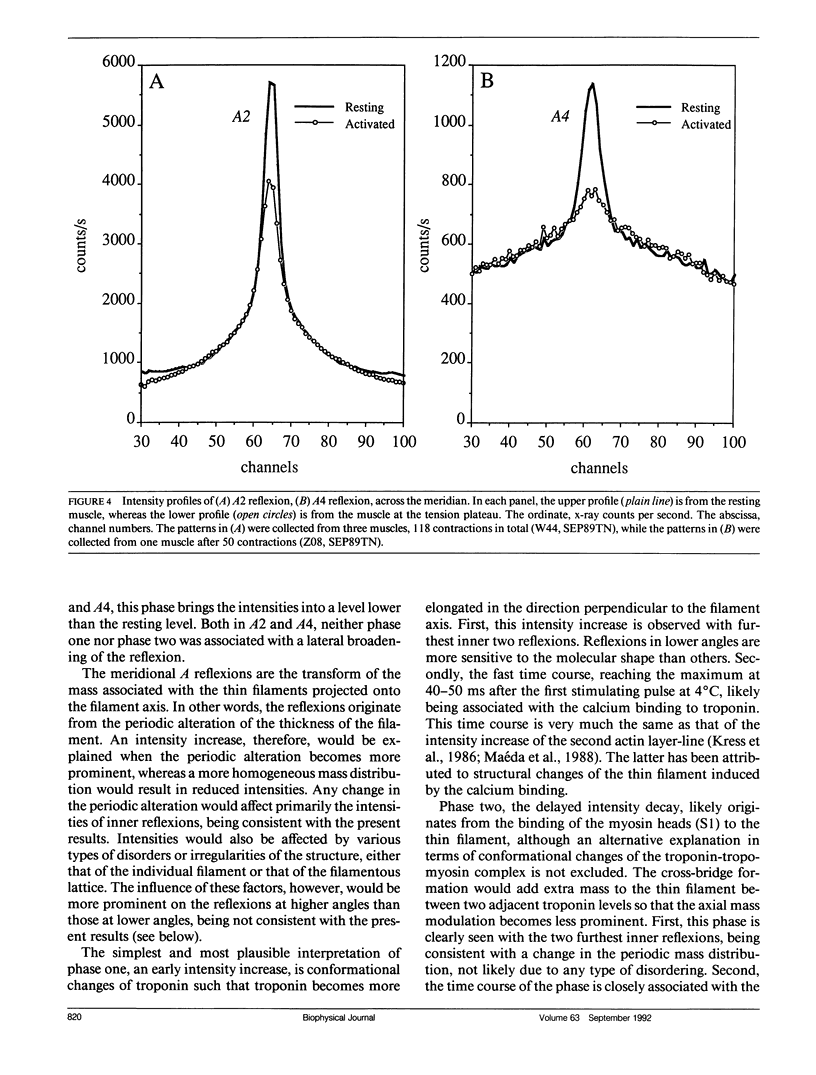
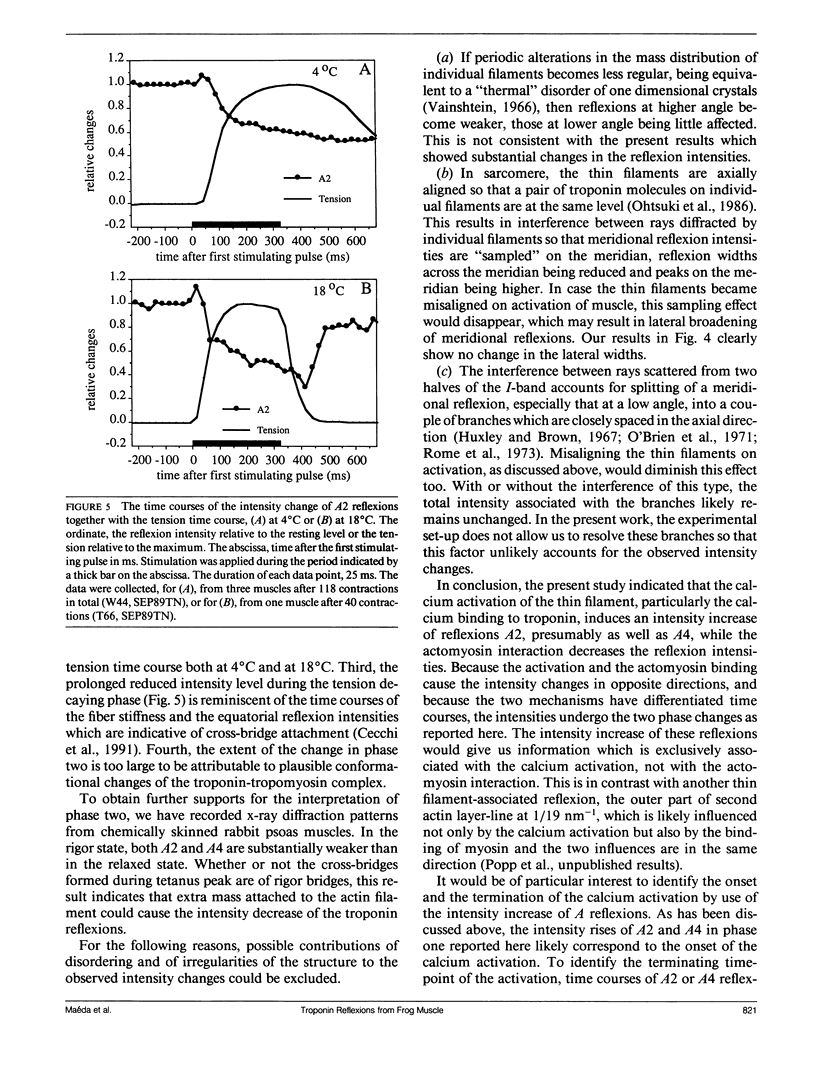
The vertebrate skeletal muscle gives rise to a series of x-ray reflexions indexed as orders (n) of 77 nm, the even orders being meridional whereas the odd orders being near-meridional. The diffraction intensities associated with these reflexions originate from the axial period of 39 nm attributable to the repeat of troponin-tropomyosin on the thin filament. In the present study, the x-ray intensities of the furthest inner reflexions, A2 (n = 2) reflexion at an axial spacing of 1/39 nm-1 and A4 (n = 4) reflexion at 1/19 nm, of this series were measured with a time resolved manner. Upon activation of the frog striated muscle, the two reflexions underwent biphasic time courses of the intensity changes. With A2 reflexion, a rapid intensity increase by 16%, being completed by the time when tension rises to 5%, was followed by a slow intensity decrease down to 50%, which was associated with the tension rise. In both phases, lateral widths remained unchanged. A4 reflexion also behaves in the same way, although the first phase (the intensity increase) was not clear due to unsatisfactory statistics. We interpret phase one as being caused by conformational change of the troponin-tropomyosin complex upon binding of Ca2+ to troponin, whereas phase two being due to direct contribution of the mass of the myosin heads bound to the thin filament, although possible contribution of conformational changes of the regulatory proteins to phase two is not excluded. The results indicated that the calcium activation of the thin filament leads the onset of the actomyosin interaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cecchi G., Griffiths P. J., Bagni M. A., Ashley C. C., Maeda Y. Time-resolved changes in equatorial x-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophys J. 1991 Jun;59(6):1273–1283. doi: 10.1016/S0006-3495(91)82342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Griffiths P. J., Potter J. D., Maéda Y., Ashley C. C. Transient kinetics and time-resolved X-ray diffraction studies in isolated single muscle fibres. Adv Exp Med Biol. 1988;226:113–128. [PubMed] [Google Scholar]
- Haselgrove J. C. X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle. J Mol Biol. 1975 Feb 15;92(1):113–143. doi: 10.1016/0022-2836(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Maéda Y., Boulin C., Gabriel A., Sumner I., Koch M. H. Intensity increases of actin layer-lines on activation of the Limulus muscle. Biophys J. 1986 Dec;50(6):1035–1042. doi: 10.1016/S0006-3495(86)83547-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maéda Y., Matsubara I., Yagi N. Structural changes in thin filaments of crab striated muscle. J Mol Biol. 1979 Jan 15;127(2):191–201. doi: 10.1016/0022-2836(79)90239-0. [DOI] [PubMed] [Google Scholar]
- Maéda Y., Popp D., McLaughlin S. M. Cause of changes in the thin filament-associated reflexions on activation of frog muscle--myosin binding or conformational change of actin. Adv Exp Med Biol. 1988;226:381–390. [PubMed] [Google Scholar]
- Maéda Y. X-ray diffraction patterns from molecular arrangements with 38-nm periodicities around muscle thin filaments. Nature. 1979 Feb 22;277(5698):670–672. doi: 10.1038/277670a0. [DOI] [PubMed] [Google Scholar]
- Namba K., Wakabayashi K., Mitsui T. X-ray structure analysis of the thin filament of crab striated muscle in the rigor state. J Mol Biol. 1980 Mar 25;138(1):1–26. doi: 10.1016/s0022-2836(80)80002-7. [DOI] [PubMed] [Google Scholar]
- O'Brien E. J., Bennett P. M., Hanson J. Optical diffraction studies of myofibrillar structure. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):201–208. doi: 10.1098/rstb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Ohtsuki I., Maruyama K., Ebashi S. Regulatory and cytoskeletal proteins of vertebrate skeletal muscle. Adv Protein Chem. 1986;38:1–67. doi: 10.1016/s0065-3233(08)60525-2. [DOI] [PubMed] [Google Scholar]
- Rome E. M., Hirabayashi T., Perry S. V. X-ray diffraction of muscle labelled with antibody to troponin-C. Nat New Biol. 1973 Aug 1;244(135):154–155. doi: 10.1038/newbio244154a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Amemiya Y., Fujishima A., Kobayashi T., Hamanaka T., Sugi H., Mitsui T. Time-resolved x-ray diffraction studies on the intensity changes of the 5.9 and 5.1 nm actin layer lines from frog skeletal muscle during an isometric tetanus using synchrotron radiation. Biophys J. 1985 Jun;47(6):847–850. doi: 10.1016/S0006-3495(85)83989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J., Vibert P., Cohen C. Actin filaments in muscle: pattern of myosin and tropomyosin/troponin attachments. J Mol Biol. 1978 Sep 25;124(3):501–521. doi: 10.1016/0022-2836(78)90184-5. [DOI] [PubMed] [Google Scholar]
- Zappe H. A., Maéda Y. X-ray diffraction study of fast and slow mammalian skeletal muscle in the live relaxed state. J Mol Biol. 1985 Sep 5;185(1):211–214. doi: 10.1016/0022-2836(85)90193-7. [DOI] [PubMed] [Google Scholar]



