Abstract
Background
The ability of transfected synthetic small interfering (si) RNAs to suppress the expression of specific transcripts has proved a useful technique to probe gene function in mammalian cells. However, high production costs limit this technology's utility for many laboratories and experimental situations. Recently, several DNA-based plasmid vectors have been developed that direct transcription of small hairpin RNAs, which are processed into functional siRNAs by cellular enzymes. Although these vectors provide certain advantages over chemically synthesized siRNAs, numerous disadvantages remain including merely transient siRNA expression and low and variable transfection efficiency.
Results
To overcome several limitations of plasmid-based siRNA, a retroviral siRNA delivery system was developed based on commerically available vectors. As a pilot study, a vector was designed to target the human Nuclear Dbf2-Related (NDR) kinase. Cells infected with the anti-NDR siRNA virus dramatically downregulate NDR expression, whereas control viruses have no effect on total NDR levels. To confirm and extend these findings, an additional virus was constructed to target a second gene, transcriptional coactivator p75.
Conclusion
The experiments presented here demonstrate that retroviruses are efficient vectors for delivery of siRNA into mammalian cells. Retrovirus-delivered siRNA provides significant advancement over previously available methods by providing efficient, uniform delivery and immediate selection of stable "knock-down" cells. This development should provide a method to rapidly assess gene function in established cell lines, primary cells, or animals.
Background
RNA interference (RNAi) is a phenomenon describing double-stranded (ds)RNA-dependent gene specific posttranscriptional silencing. Initial attempts to harness this phenomenon for experimental manipulation of mammalian cells were foiled by a robust and nonspecific antiviral defense mechanism activated in response to long dsRNA molecules [1]. The field was significantly advanced upon the demonstration that synthetic duplexes of 21 nucleotide RNAs could mediate gene specific RNAi in mammalian cells, without invoking generic antiviral defense mechanisms [2,3]. As a result, siRNAs have become powerful tools to dissect gene function. Unfortunately, the chemical synthesis of small RNAs is prohibitively expensive for numerous laboratories and experimental situations. Consequently, numerous groups have sought the development of DNA-based vectors capable of generating such siRNA within cells. Several groups have recently attained this goal and published similar strategies that, in general, involve transcription of short hairpin (sh)RNAs that are efficiently processed to form siRNAs within cells [4-7]. These reports describe intricate (and ingenious) methods to generate siRNAs capable of specifically targeting numerous endogenously and exogenously expressed genes. With time, these substantial advances will undoubtedly provide critical insight into gene function.
Despite the advantages of these new plasmid vectors, numerous shortcomings remain. In particular, the expression of the siRNA is transient and, like any other plasmid, the transfection efficiency is often low and inconsistent. Therefore, we sought to develop a more tractable and efficient siRNA delivery system. The following brief report describes a method to incorporate the PolIII-transcribed small hairpin system into a retroviral vector. Our results suggest retroviruses will be extremely useful delivery vehicles for siRNA expression in mammalian cells.
Results
Development of a retroviral siRNA delivery vector
Recently, several DNA-based RNAi vectors have become available [4-7]. We initially employed the pBS/U6 vector (now sold as pSilencer 1.0-U6, available from Ambion, Austin, TX) which contains the RNA PolIII-specific U6 gene promoter along with restriction sites convenient for oligonucleotide insertion. [6]. We chose to target a poorly defined human serine-threonine kinase, Nuclear Dbf-2 Related (NDR). Oligonucleotides targeting a region of NDR were cloned into the pBS/U6 vector to generate pBS/U6-NDR. When HeLa cells were transiently transfected with pBS/U6-NDR, no noticeable decrease in NDR expression was detectable by western blot (data not shown). Interpretation of the above experiment was complicated due to the relatively high number of untransfected HeLa cells still expressing normal levels of NDR.
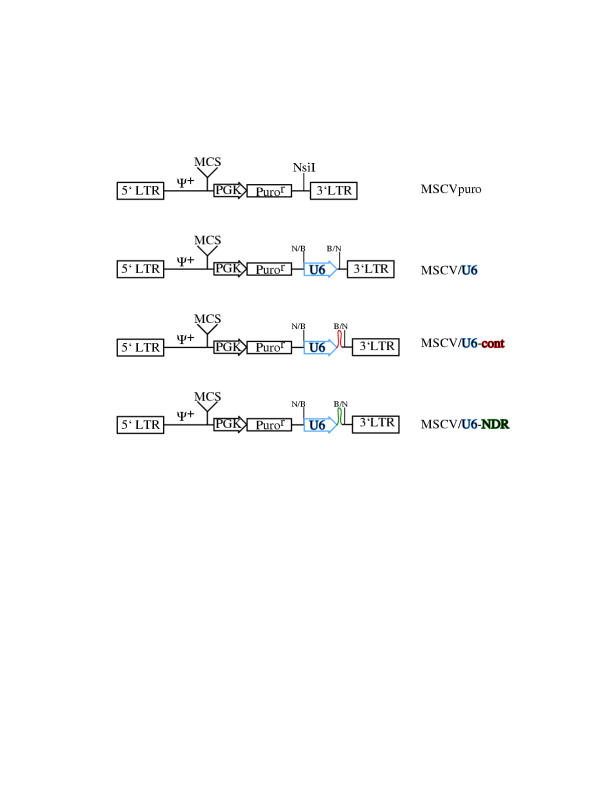
To circumvent the problems associated with transient transfections, a retroviral siRNA vector was developed. As illustrated in Figure 1, the U6 promoter and anti-NDR hairpin was subcloned from pBS/U6-NDR into pMSCVpuro (Clontech, Palo Alto, CA) at the unique NsiI site just upstream from the 3' LTR. Retroviral vectors containing only the U6 promoter (pMSCV/U6) or containing the U6 promoter plus an irrevelant hairpin (pMSCV/U6-cont) were also constructed.
Figure 1.
Construction of retroviral siRNA delivery vector Schematic of genome structure. All plasmids were derived from pMSCVpuro (Clontech). The inserted U6 promoter is illustrated in blue, while the control and anti-NDR hairpins are shown in red and green, respectively. Ψ+, extended packaging signal. MCS, multiple cloning site. PGK, murine phosphoglycerate kinase promoter. Puror, puromycin resistance gene. N/B and B/N, cloning junction generated by ligating the blunt ended BamHI and NsiI restriction sites.
Stable, efficient inhibition of NDR by retrovirus-delivered siRNA
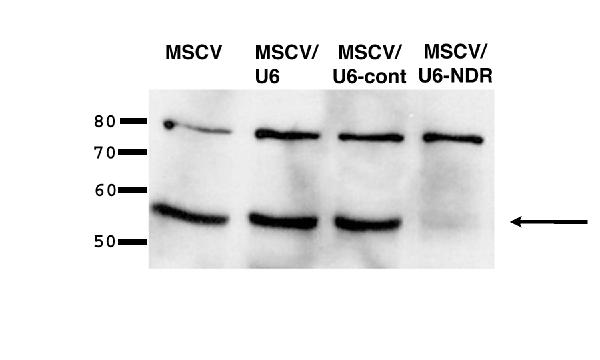
HeLa cells were mock-infected or infected with MSCVpuro, MSCV/U6, MSCV/U6-cont, or MSCV/U6-NDR. Twenty-four hours after infection, infected cells were selected in media containing puromycin. After a single day of selection, all mock-transfected cells died, whereas nearly all cells infected with MSCV, MSCV/U6, MSCV/U6-cont, or MSCV/U6-NDR survived and proliferated. These results indicate that insertion of the U6 promoter upstream of the 3' LTR has no noticeable effects on virus packaging or infectivity. After expanding the infected cell population, a fraction of the cells were lysed for western blot analysis. An equal amount of total protein was fractionated by SDS-PAGE and probed with monoclonal antibodies to NDR (~54 KDa) and an unrelated protein (p75) as a loading and specificity control (Fig. 2). The western blot demonstrates that a dramatic downregulation of NDR occurs following infection with MSCV/U6-NDR but is unchanged upon infection with control viruses. Cells lysed 24 hours post-infection (ie., without selection) gave identical results, indicating the integrated virions induce rapid NDR silencing (data not shown). The HeLa population infected with MSCV/U6-NDR has maintained NDR silencing after several passages (data not shown).
Figure 2.
Rapid, stable suppresion of NDR by retrovirus-delivered siRNA HeLa cells were infected with the viruses illustrated in Fig. 1. Proteins were extracted from cells infected with each virus and NDR levels were examined by western blot analysis. Arrow indicates dramatic downregulation of NDR only in cells infected with MSCV/U6-NDR. The upper band was detected by the anti-p75 antibody, which serves as an internal loading and specificity control. Identical results were obtained with cells examined 24-hours post infection or after several passages (data not shown).
Stable, efficient inhibition of p75 by retrovirus-delivered siRNA
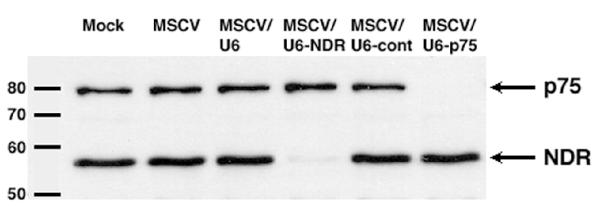
We next sought to confirm and extend the above findings by generating a retrovirus targeting an additional gene, transcriptional coactivator p75. HeLa cells obtained from an alternative source (in this case, HeLa-CD4 cells) were infected and selected essentially as described above. Western blot analysis demonstrates a remarkable downregulation of p75 occurs specifically in cells infected with MSCV/U6-p75 (Fig. 3). Likewise, levels of NDR are dramatically reduced in cells infected with MSCV/U6-NDR (Fig. 3).
Figure 3.
Suppression of NDR and p75 expression by retrovirus-delivered siRNA. HeLa-CD4 cells were infected the viruses illustrated in Fig. 1 plus an additional virus, MSCV/U6-p75. Western blot analysis demonstrates a dramatic downregulation of p75 in cells infected with MSCV/U6-p75 and NDR in cells infected with MSCV/U6-NDR. Infection with the various control viruses (MSCV, MSCV/U6, or MSCV/U6-cont) had no effect on p75 or NDR levels. For the mock-infected sample, puromycin selection was omitted.
Discussion
Several strategies to express siRNA via DNA-based plasmids have recently become available. While these plasmids provide a significant advantage over synthetic RNAs, several disadvantages remain, including all of the well-known difficulties involving transient transfections (low efficiency, uneven distribution, high variability, etc.). As a result, determining the effectiveness of a particular vector is not a trivial endeavour, particular since the "effect" may be a modest gene downregulation within a small fraction of total cells. Moreover, with both synthetic siRNA and plasmid-based vectors, the siRNA expression is transient. Selection of stable clones has been demonstrated with plasmid vectors, although this process requires several weeks or months and is ultimately problematic due to potential clonal effects.
To overcome these problems, we sought to incorporate the key elements of the well-defined pBS/U6 siRNA vector into a retrovirus. Data presented in this report indicate that retroviruses are suitable vectors to deliver siRNAs to mammalian cells. Given the simplicity of this system, which employs readily available reagents, any laboratory should be able to initiate a retroviral siRNA project. We have identified a viable insertion site for an RNA PolIII promoter and oligonucleotide hairpin into a retrovirus that does not noticeably affect packaging or infectivity. By inserting the U6 promoter/hairpin at this site, we have preserved the multiple cloning site (MCS) of pMSCVpuro (Fig 1), which could allow insertion of an additional gene of interest for knock-in/knock-down experiments. Using this strategy, we have generated retroviruses that efficiently downregulate the expression of the protein kinase NDR and the transcriptional coactivator p75 by RNAi. The stable propagation of these cell lines demonstrates that "wild-type" levels of NDR or p75 are not essential for HeLa cell viability. It is worth noting that although the levels of NDR and p75 are dramatically reduced following infection with the respective viruses, the resultant cells may not be true "knock-outs."
The methods described above involve initially subcloning the hairpin into a transition vector (pBS/U6, now sold as pSilencer 1.0-U6) prior to insertion into pMSCVpuro. Slight modifications of pMSCVpuro (or pMSCV/U6 vector developed here) will allow single one-step cloning of oligonucleotide duplexes. However, the initial cloning step may prove useful in many cases, as it may provide unique restriction sites for rapid insertion into other retroviral vectors containing convenient markers (i.e. alternate antibiotic resistance markers, GFP) or other context-specific features (ie., knock-in Gene A, knock-down Gene B). It is also enticing to envision retroviral-delivered regulatable RNAi. Indeed, the U6 promoter has been successfully modified by incorporation of tetracycline (Tet)-operator sites [8]; this may prove useful when studying "essential" genes. Once engineered into a retrovirus, this system could be used to study early-stage events associated with knocking-out a specific gene, such as changes in gene expression, protein localization, or cellular architecture.
Conclusions
The current work has combined two well-described and commercially available systems. Our results demonstrate that retroviruses are viable vectors for delivery of siRNAs. This approach represents a dramatic improvement over plasmid-based delivery systems, as retroviral infection is rapid, uniform, and stable. The function of virtually any gene can now be assayed in virtually any cell type, presumably including non-dividing primary cells if a lentivirus packaging system is employed. Numerous improvements to the present system can be envisioned, some of which are highlighted above. As this technology continues to evolve, researchers will be able to address previously impenetrable questions in cell biology.
Methods
Construction of a retroviral vector for delivery of siRNA
The general strategy described for oligonucleotide insertion into pBS/U6 [[6], Yang Shi, personal communication] was employed with some modification. Specifically, the cloning was reduced to a single-step process, rather than the piece-wise construction reported by Shi and colleagues. To generate pBS/U6-NDR, two oligonucleotides were synthesized.
Oligo 1 (top-strand):
5'-GGACATGATGACCTTGTTGAaagcttTCAACAAGGTCATCATGTCCCTTTTTG-3'.
Oligo 2 (bottom strand):
5'-AATTCAAAAAGGGACATGATGACCTTGTTGAaagcttTCAACAAGGTCATCATGTCC-3'.
The first stretch of underlined nucleotides correspond to nucleotides 515–535 of the NDR open reading frame (nucleotides 1111–1130 of the NDR mRNA sequence, GenBank accession number NM_007271.). The lower case letters represent a HindIII site. The second stretch of underlined nucleotides is the reverse complement of the first; transcribed RNA is therefore predicted to form a small hairpin. The two oligonucleotides were annealed and inserted into pBS/U6 digested with ApaI (and subsequently filled in with Klenow) and EcoRI. Clones containing the oligonucleotide insertion can be distinguished from pBS/U6 by a diagnostic BamHI/XhoI digest (the XhoI site present in the pBS/U6 MCS is lost upon insertion of the oligonucleotide; clones with an oligonucleotide insert will thus liberate a larger BamHI-BamHI fragment). Verification of the inserted sequence by automated DNA sequencing is not possible, presumably due to the strong secondary structure associated with the short hairpin. Inclusion of a larger "loop" (instead of the palindromic HindIII sequence) could potentially overcome this problem.
pBS/U6-NDR was digested with BamHI to liberate the U6 promoter and the oligonucleotide region complementary to NDR. This BamHI-BamHI fragment was subsequently blunt ended with Klenow and inserted into pMSCVpuro (Clontech, Palo Alto, CA; vector sequence available at http://www.clontech.com) blunt ended at the unique NsiI site, generating pMSCV/U6-NDR. The BamHI-BamHI fragment from pBS/U6, containing the U6 promoter and MCS, was inserted into pMSCVpuro to generate pMSCV/U6. An additional plasmid containing an irrelevant hairpin was similarly constructed to generate pMSCV/U6-cont. For consistency, only plasmids with the U6 promoter pointing toward the 3' LTR were chosen for further study (see Fig. 1). The above strategy was also used to generate pMSCV/U6-p75 with the following oligonucleotides:
Oligo 1 (top-strand):
5'-GGTCAAAGACTCTAAATGGAGaagcttCTCCATTTAGAGTCTTTGACCCTTTTTG-3'
Oligo 2 (bottom strand):
5'-aattcaaaaaGGGTCAAAGACTCTAAATGGAGaagcttCTCCATTTAGAGTCTTTGACC-3'
The underlined sequence corresponds to nucleotides 1420–1441 of the p75 open reading frame (GenBank accession number AF063020).
Viral packaging
pMSCVpuro, pMSCV/U6, pMSCV/U6-cont, or pMSCV/U6-NDR were packaged in Pheonix Ampho cells (a kind gift of G. Nolan, Standford University) by standard calcium phosphate transfection [9]. To improve titer, additional Gag-Pol and the VSV-G envelope was expressed by cotransfection with PCG-gagpol and PCG-VSV-G (kind gifts of Jonathon Walsh and Richard Mulligan, Harvard Medical School). Virus supernatant was collected 48 hours post-transfection, pelleted to remove nonadherent cells and cellular debris, frozen in small aliquots on dry ice, and stored at -80 C.
HeLa cell infection and selection
Twenty-four hours prior to infection, HeLa cells (kind gift of Tom Roberts, Harvard Medical School) were seeded at 20,000 cells per well in 24-well plates. The following day, the culture media was aspirated and replaced with virus supernatant diluted 1:2 (1 ml final) in growth media (DMEM, 10% Fetal Bovine Serum, Penicillin-Streptomycin). Polybrene was added to a final concentration of 4 ug/ml. 24 hours after infection, the cells were collected by trypsinization and reseeded in 6 well dishes in selective media (growth media + 1 ug/ml puromycin). By the next day, all mock-infected cells were floating and presumably dead or dying. Nearly all cells infected with MSCV-puro, MSCV/U6, MSCV/U6-cont, or MSCV/U6-NDR adhered to the wells and began to proliferate. In a subsequent experiment, HeLa-CD4 cells (a kind gift of Alan Engelman, Harvard Medical School) were seeded at 200,000 cells per well in a 6-well plate and infected with 2 ml of diluted (1:2) virus supernatant. One day after infection, the cells were collected and reseeded in 10-cm plates in selective media. Virtually all of the cells subsequently adhered to the new dish (with the exception of the mock control), suggesting that viral titers were at least 2 × 105/ml.
Protein extraction and western blot analysis
Protein was extracted in ice-cold cell lysis buffer (50 mM Tris pH 7.5, 300 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, Roche Complete Protease Inhibitor). Protein concentration was determined using the BioRad DC Assay kit. Fifteen μg of total protein was resolved by SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal anti-NDR and monoclonal anti-p75 (both from Transduction Laboratories; Lexington, KY). Amido black staining of the membrane further verified equivalent loading and even electrotransfer (data not shown).
Authors' contributions
E.D. developed the retroviral siRNA system, performed the described experiments, and drafted the manuscript. P.S is the Prinicipal Investigator of the lab and participated in this study's design and development.
Acknowledgments
Acknowledgements
This work was supported by grants from the NIH and the Claudie Adams Barr Fund (P.S) and an HHMI Predoctoral Fellowship (E.D.). The authors wish to thank Yang Shi, Jonathon Walsh, and Richard Mulligan for plasmids, Gary Nolan for Pheonix Ampho cells, Tom Roberts for HeLa cells, Alan Engelman for HeLa-CD4 cells, and Alan Engelman and Guillaume Adelmant for insightful discussions.
Contributor Information
Eric Devroe, Email: devroe@fas.harvard.edu.
Pamela A Silver, Email: pamela_silver@dfci.harvard.edu.
References
- Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): Mechanisms of action. Apoptosis. 2000;5:107–114. doi: 10.1023/A:1009664109241. [DOI] [PubMed] [Google Scholar]
- Elbashir S, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Caplen N, Parrish S, Imani F, Fire A, Morgan R. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate cells. Proc Natl Acad Sci. 2001;98:9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison P, Caudy A, Hannon G. Stable suppresion of gene expression by RNAi in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:1443–1448. doi: 10.1073/pnas.032652399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddison P, Caudy A, Bernstein E, Hannon G, Conklin D. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes & Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar E, Gay F, Shi Y, Forrester W, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci. 2002;8:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelkamp T, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Ohkawa J, Taira K. Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum Gene Ther. 2000;11:577–585. doi: 10.1089/10430340050015761. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fitsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Press. 1989.