Abstract
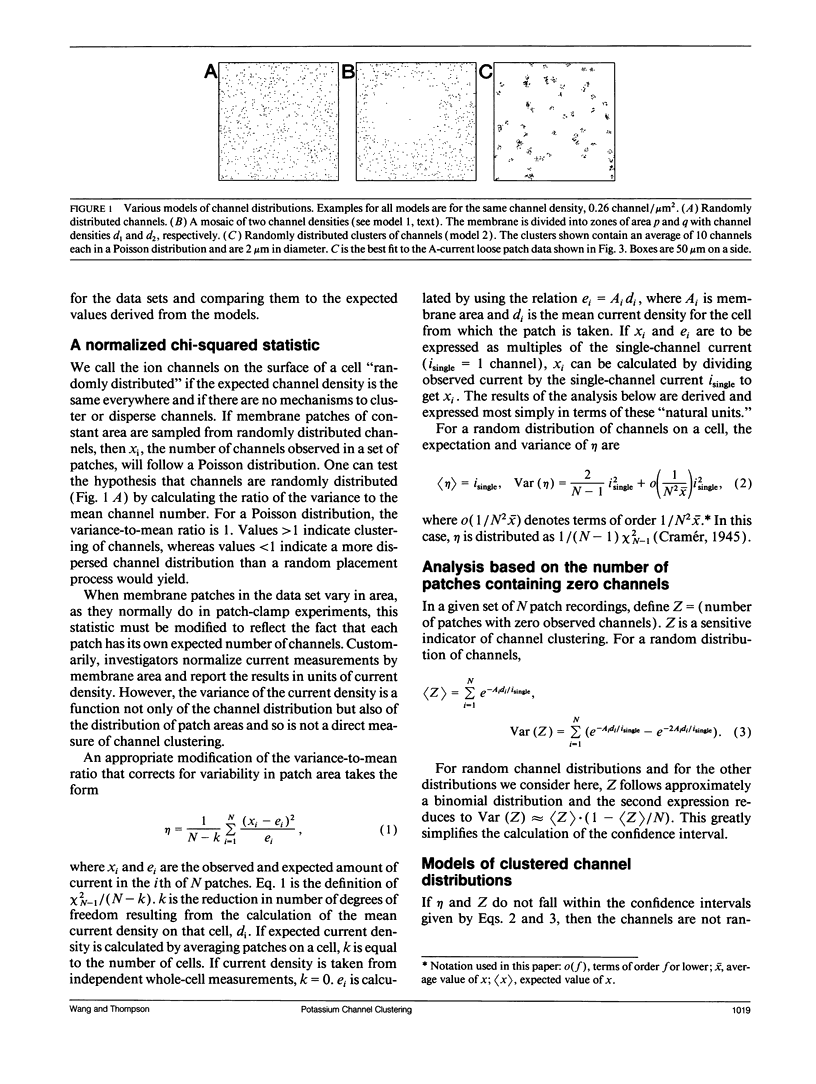
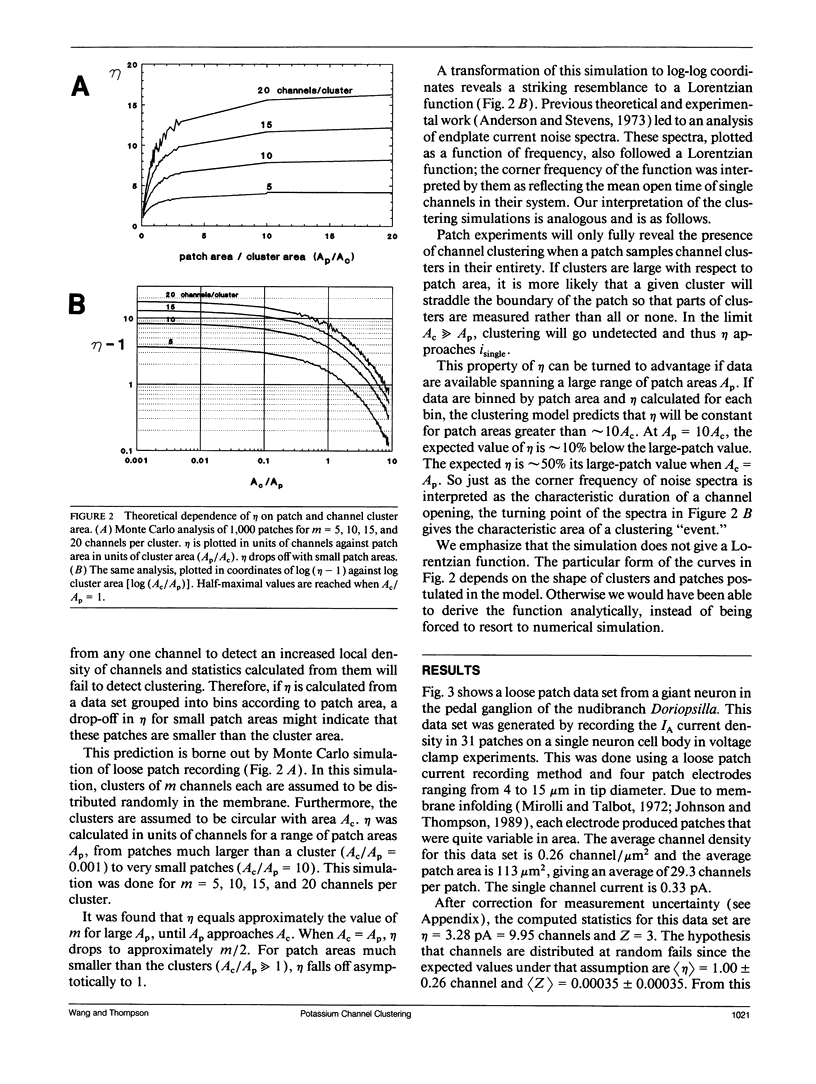
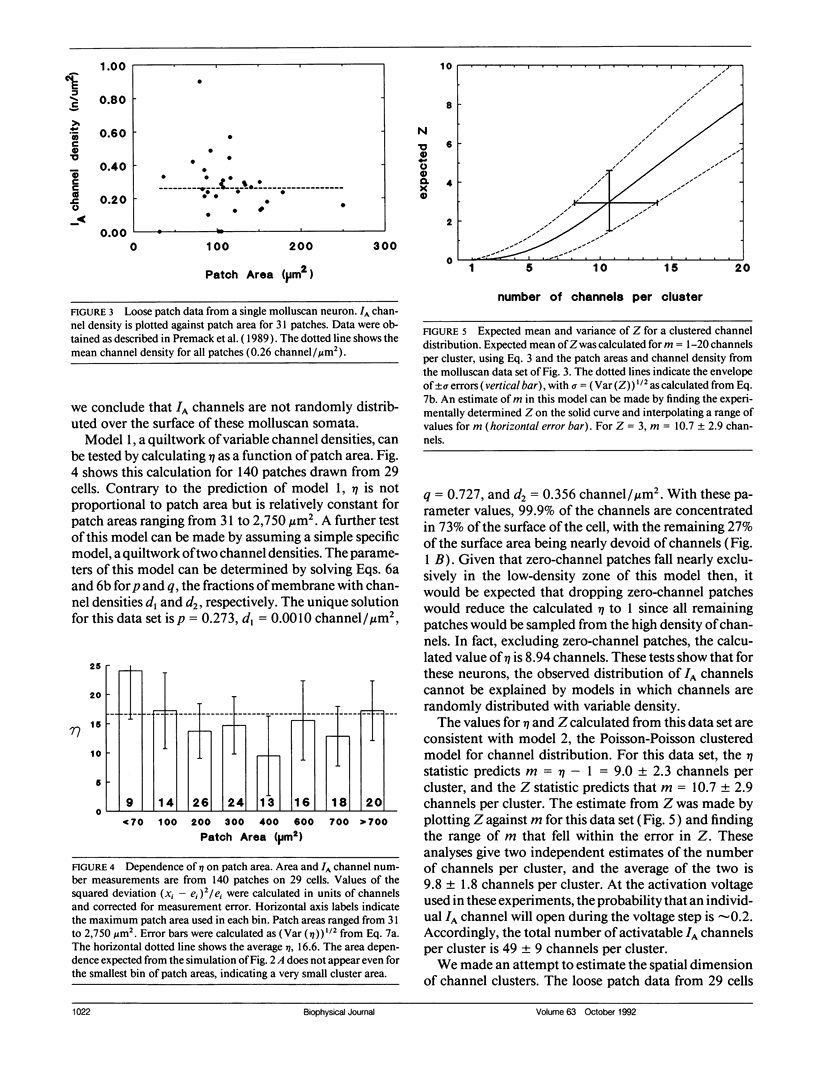
The spatial distribution of ion channels over the surface of a neuron is an important determinant of its excitable properties. We introduce two measures of channel clustering for use in patch-clamp experiments: a normalized chi-squared statistic (eta) and the number of zero-channel patches in a data set (Z). These statistics were calculated for data sets describing the distribution of A-type potassium channels on neurons of the nudibranch Doriopsilla and measurements of Ca-dependent outward current channels on bullfrog hair cells, as well as simulated channel distributions. When channels are clustered, eta is approximately equal to the amount of current in a cluster. The analysis shows that somatic A-channels in the nudibranch are distributed in clusters of approximately 50 channels each. The clusters are < 2 microns wide and are separated, on average, by 3.2 microns. Outward current channels on hair cells occur in clusters of approximately 27 channels each, in agreement with the original analysis. Channel clustering may reflect properties of the insertion or regulation of channels in the membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stirling C. Distribution of transport proteins over animal cell membranes. J Membr Biol. 1984;77(3):169–186. doi: 10.1007/BF01870567. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides K. J., Elmer L. W., Loftus D., Elson E. Distribution and lateral mobility of voltage-dependent sodium channels in neurons. J Cell Biol. 1988 Jun;106(6):1911–1925. doi: 10.1083/jcb.106.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides K. J. Fluorescently labelled Na+ channels are localized and immobilized to synapses of innervated muscle fibres. Nature. 1986 May 1;321(6065):63–66. doi: 10.1038/321063a0. [DOI] [PubMed] [Google Scholar]
- Beam K. G., Caldwell J. H., Campbell D. T. Na channels in skeletal muscle concentrated near the neuromuscular junction. Nature. 1985 Feb 14;313(6003):588–590. doi: 10.1038/313588a0. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Localization of sodium channels in cultured neural cells. J Neurosci. 1981 Jul;1(7):777–783. doi: 10.1523/JNEUROSCI.01-07-00777.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Levinson S. R. Immunocytochemical localization of sodium channel distributions in the excitable membranes of Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6707–6711. doi: 10.1073/pnas.79.21.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Thompson S. Measurement of nonuniform current densities and current kinetics in Aplysia neurons using a large patch method. Biophys J. 1989 Feb;55(2):299–308. doi: 10.1016/S0006-3495(89)82805-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Mirolli M., Talbott S. R. The geometrical factors determining the electrotonic properties of a molluscan neurone. J Physiol. 1972 Dec;227(1):19–34. doi: 10.1113/jphysiol.1972.sp010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. M. Mobility and localization of proteins in excitable membranes. Annu Rev Neurosci. 1985;8:369–406. doi: 10.1146/annurev.ne.08.030185.002101. [DOI] [PubMed] [Google Scholar]
- Premack B. A., Thompson S., Coombs-Hahn J. Clustered distribution and variability in kinetics of transient K channels in molluscan neuron cell bodies. J Neurosci. 1989 Nov;9(11):4089–4099. doi: 10.1523/JNEUROSCI.09-11-04089.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Almers W. Photobleaching through glass micropipettes: sodium channels without lateral mobility in the sarcolemma of frog skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):946–950. doi: 10.1073/pnas.79.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Coombs J. Spatial distribution of Ca currents in molluscan neuron cell bodies and regional differences in the strength of inactivation. J Neurosci. 1988 Jun;8(6):1929–1939. doi: 10.1523/JNEUROSCI.08-06-01929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Topographical rearrangement of acetylcholine receptors alters channel kinetics. Nature. 1983 Jul 14;304(5922):161–163. doi: 10.1038/304161a0. [DOI] [PubMed] [Google Scholar]


