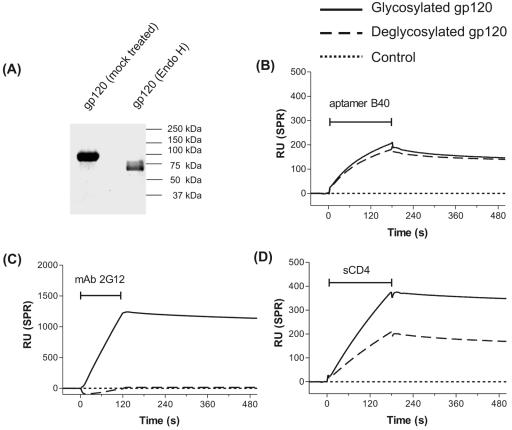
FIG. 1.
The binding of aptamer to gp120 is independent of N-linked glycosylation. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the endo H-treated deglycosylated gp120. The molecular mass (∼63 kDa) of the endo H-treated gp120, which is reduced compared to that of the mock-treated gp120 (∼92 kDa) indicates near-complete deglycosylation of the native glycoprotein. (B-D) Overlay of BIAcore sensorgrams to show the binding of the indicated analytes to glycosylated (solid line) and deglycosylated (dashed line) Ba-L monomeric gp120 immobilized on a CM5 biosensor chip. The signal from a control cell for each analyte (dotted line) has been subtracted from all sensorgrams in this and subsequent figures. The horizontal I-shaped bars show the injection period of the analytes. RU, response units; SPR, surface plasmon resonance.