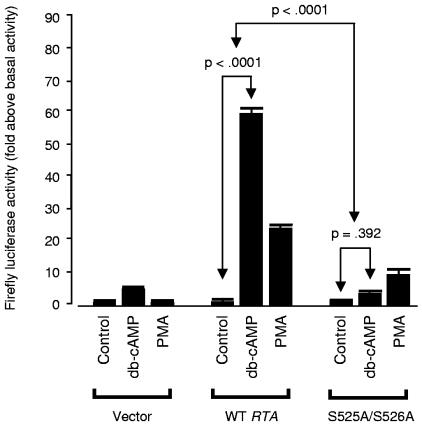
FIG. 5.
Role of RTA serines 525 and 526 in PKA-mediated enhancement of trans-activation. To evaluate the functional role of predicted phosphorylation targets at S525 and S526 of RTA, both residues were converted to alanines through PCR-based site-directed mutagenesis. RTA's trans-activating capacity was assayed using a luciferase reporter driven by the KSHV PAN promoter as in Fig. 4B. Reporter constructs were electroporated into uninfected Ramos cells accompanied by either 1 μg of pFLAG/RTA (WT RTA), 1 μg of pFLAG/RTA-S525A-S526A (mutant), or 1 μg of empty pFLAG (Vector). Cells were subsequently cultured in the presence of 300 μM db-cAMP or 20 ng/ml PMA as indicated, and Firefly luciferase activity was measured 18 h later as a fold change above basal activity after normalization to control Renilla luciferase levels. Values represent the mean (± standard error) fold increase in PAN promoter activity relative to the vehicle-treated control for each construct (Vector, WT RTA, S525A-S526A mutant RTA). Similar results emerged when data were normalized to a common basal condition (e.g., vehicle-treated cells electroporated with the empty vector). In similar studies using KS-1 cells and norepinephrine, endogenous regulation of RTA expression complicated analyses but data continued to show greater PKA-induced up-regulation of RTA activity on the PAN promoter for wild-type RTA versus the S525A-S526A mutant (results reported in main text).