Figure 5.
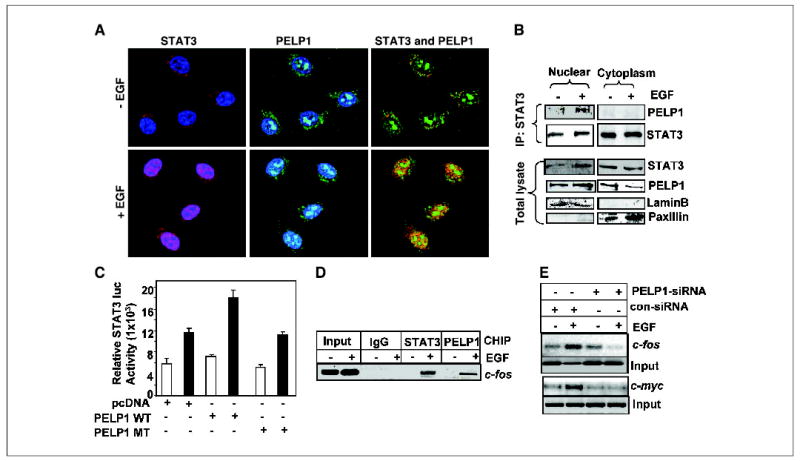
PELP1 and STAT3 colocalize in the nuclear compartment. A, MCF-7 cells were treated with or without EGF (100 ng/mL). The cells were then fixed with methanol and costained with antibodies against PELP1 (green) and STAT3 (red). Blue, DNA stained with Topo-3. The images were analyzed by confocal microscopy. Yellow, colocalization of PELP1 and STAT3. B, MCF-7 cells were treated with or without EGF and biochemically fractionated into nuclear and cytoplasm extracts. The total lysates from each compartment were subjected to immunoprecipitation with a STAT3 antibody followed by immunoblotting with PELP1. Lamin B and paxillin were used as markers of the nuclear and cytoplasm compartments. C, MCF-7 cells were transfected with the STAT3 reporter gene along with wild-type PELP1 or mutant PELP1, which lacks a histone binding domain. After 24 hours, the cells were serum starved and treated with EGF, and the STAT3 reporter activity was determined. D, MCF-7 cells expressing T7-PELP1 were treated with EGF and cross-linked with formaldehyde, and the chromatin was immunoprecipitated with control immunoglobulin G (IgG), STAT3, and T7 epitope antibodies. DNA eluates from the chromatin precipitates were used to determine the recruitment of STAT3 and PELP1 to the STAT3-responsive element–containing region of the c-fos promoter by PCR. E, MCF-7 cells were transfected with control or PELP1-specific siRNA. After 48 hours, the cells were serum starved for 24 hours and treated with EGF (100 ng/mL) for 1 hour. Chromatin was isolated and immunoprecipitated with STAT3 antibody. STAT3 recruitment to the target gene promoters (c-fos and c-myc) was analyzed by PCR.
