Abstract
Signaling pathways mediating the divergent effects of FSH and LH on aromatase in immature rat granulosa cells were studied by infecting cells with increasing amounts of adenoviral vectors for the hLHR or hFSHR. Increasing amounts of Ad-hLHR, used at a multiplicity of infection (MOI) of 20 or 200 viable viral particles/cell increased hCG binding, hCG-induced cAMP and Akt phosphorylation but inositol phosphates only increased in response to hCG in cells infected with 200 MOI Ad-hLHR. In contrast hCG increased aromatase expression in cells infected with 20 but not in cells infected with 200 MOI Ad-hLHR. Cells infected with 20 or 200 MOI Ad-hFSHR showed increased hFSH binding and hFSH-induced Akt phosphorylation, but the hFSH-induced cAMP response was unchanged relative to control cells. However, hFSH was able to stimulate the inositol phosphate cascade in the Ad-hFSHR infected cells, and the hFSH induction of aromatase was abolished. We also found that activation of C kinase or expression of a constitutively active form of Gαq inhibited the induction of aromatase by hFSH or 8Br-cAMP.
We conclude that the differential effects of FSH and LH on aromatase in immature granulosa cells are highly dependent on gonadotropin receptor density and on the signaling pathways activated. We propose that aromatase is induced by common signals generated by activation of the FSHR and LHR (possibly cAMP and Akt) and that the activation of the inositol phosphate cascade in cells expressing a high density of LHR or FSHR antagonizes this induction.
Introduction
The development of ovulatory follicles involves the differentiation of their granulosa cell component from a mitotically active, estrogen-secreting type (immature cells) into a non-dividing, luteinized, progesterone-secreting type (mature cells). Granulosa cell differentiation is regulated by two pituitary hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH) as well as intra-ovarian steroids and growth factors (reviewed in refs. 1, 2, 3).
The receptors for FSH (FSHR) and LH (LHR) are members of the G-protein coupled family of receptors (GPCR) and their expression in granulosa cells depends on the stage of cell differentiation. The FSHR is expressed in both immature and mature cells, but the LHR is expressed only in the mature cell type. In immature granulosa cells, FSH promotes cell multiplication and induces the expression of aromatase and the LHR. In contrast, in mature cells the ovulatory LH surge results in cell cycle arrest, suppression of aromatase and LHR expression, increased progesterone synthesis and luteinization. These divergent effects of LH and FSH stand in contrast with the high degree of amino acid sequence homology between the two hormones (4–6) and between their two receptors (6–10), and with the fact that both the LH/LHR and the FSH/FSHR complexes use the Gs/adenylyl cyclase/cAMP as their main signaling pathway (6–10).
The reasons for the divergent actions of the LH/LHR and the FSH/FSHR pairs on granulosa cells in spite of their structural and functional similarities are not fully understood. Explanations put forward to explain these divergent actions include the expression of the LHR and FSHR at different stages of granulosa cell differentiation, differences in the properties (magnitude, duration, compartmentalization, etc) of the common cAMP signal, or differences in the generation of other intracellular signals by the two hormone/receptor pairs (2, 3, 11, 12). On the other hand, the differential effects of FSH and LH may simply reflect differences in hormone levels or in receptor density. For example, in FSH-primed immature rat granulosa cells sub-ovulatory doses of LH can stimulate estrogen synthesis and LHR expression in a manner similar to FSH (13, 14). Moreover, in estrogen-primed rats high doses of FSH can have the same effects as those seen with an ovulatory LH surge, i.e., induction of ovulation and luteinization (15).
An important technical advance in the understanding of the divergent actions of LH and FSH in the ovary is the use of adenoviral vectors to induce expression of the recombinant hLHR in primary cultures of immature rat granulosa cells. Using this experimental approach it has been shown that two of the hallmark responses of FSH action (i.e., the induction of aromatase and the LHR) are likely due to differences in the signaling properties of the LHR and the FSHR rather than to their expression at different stages of maturation of the granulosa cells (16, 17). It has also been concluded that the ability of FSH to stimulate aromatase and LHR expression involves the activation of the PKB/Akt signaling pathway (17) in addition to the cAMP pathway (1–3, 11, 12, 18–23).
In the experiments presented here we used adenovirus-directed expression of hLHR and hFSHR in primary cultures of immature rat granulosa cells to determine the receptor-activated pathways that may account for the divergent effects of FSH and LH in granulosa cells and to determine the effects of receptor density on the signaling properties of each receptor. We tested the hypothesis that the differential effects of FSH and LH on aromatase expression are due to the activation of a common stimulatory signaling pathway involving cAMP and an inhibitory signaling pathway that involves inositol phosphates and is uniquely activated by LH. Rationale for this comes from the observations that, (a) addition of hCG to cells expressing a high density of the LHR is able to stimulate not only cAMP but also inositol phosphates (reviewed in ref. 24); and (b) pharmacological activation of protein kinase C (PKC) antagonizes the effects of FSH or cAMP on the expression of aromatase and several other ovarian genes (25–28).
In doing these experiments we also found, unexpectedly, that the functional properties of the FSHR are modified by the co-expression of increasing densities of the LHR.
Materials and Methods
Plasmids, viruses and cells
The myc-tagged versions of the hLHR (29) and hFSHR (30) were subcloned by us into the RAPAd™ adenoviral vector. A constitutively active mutant of Gαq (Q209L) that has reduced capacity to hydrolyze GTP (31–33) was obtained from the Guthrie Research Center (http://www.guthrie.org/AboutGuthrie/Research/CDNA/) and subcloned in the same vector. The recombinant adenoviral particles (Ad-hLHR, Ad-hFSHR and Ad-GqQ209L) as well as recombinant adenoviral particles coding for β–galactosidase (Ad-βgal) were prepared by the Gene Transfer Vector Core of the University of Iowa (http://www.uiowa.edu/~gene/vectors.htm).
Granulosa cells from immature (25-days old) female Sprague-Dawley rats that had been treated with diethylestilbestrol (DES; 2 mg/day, injected subcutaneously in 200 μl of sesame oil) for 4 days were collected by ovarian puncture as described by others (34). These procedures were approved by the Institutional Animal Care and Use Committee for the University of Iowa. Cells were plated in 24-well plates (0.25 million cells/well) or 12-well plates (0.5 million cells/well) that had been pre-coated with bovine fibronectin. The cells were incubated at 37 C in culture medium consisting of DMEM/F12 medium (1:1, vol:vol) containing 10 mM HEPES, 50 μg/ml Gentamicin, 0.1% bovine serum albumin and supplemented with insulin (1 μg/ml), transferrin (1 μg/ml) and selenium (1 ng/ml ; ITS), as described elsewhere (20, 35). Two days after plating, the cells were incubated in culture medium without bovine serum albumin and ITS and containing the recombinant adenovirus for 2 hours at 37C. Cells infected with Ad-βgal at a multiplicity of infection (MOI) of 200 were used as controls. Other experimental groups included cells infected with Ad-hLHR or Ad-hFSHR at an MOI of 200, and cells infected with a mixture of the Ad-hLHR or Ad-hFSHR (at 20 MOI) and Ad-βgal (at 180 MOI). These conditions allowed us to keep a constant viral load while changing the amount of recombinant receptors expressed. When needed, the Ad-GqQ209L construct was used (0.5 MOI) in combination with Ad-βgal (199.5 MOI). At the end of the two-hour incubation with the viral particles the virus-containing medium was removed and replaced with culture medium. In all cases, infected cells were incubated at 37 C for two days before use in the assays described below. The expression of the recombinant receptors was shown to be optimal or near optimal after this 2-day incubation (data not presented).
Binding Assays
Cells in 12-well plates were washed and placed in 0.5 ml of assay medium (DMEM/F12 medium (1:1, vol:vol) containing 10 mM HEPES, 50 μg/ml Gentamicin, and 0.1% bovine serum albumin). Duplicate wells were incubated with 125I-hCG or 125I-FSH (100 ng/ml) for 4 h at 37 C. Nonspecific binding was determined by incubating one additional well with the same concentration of 125I-hCG or 125I-FSH plus crude hCG (50 IU/ml) or eFSH (5 μg/ml), respectively. After incubation, cells were washed two times with assay medium, lysed with 50 μl of 0.5 N NaOH, and then collected with a cotton swab and counted in a γ-counter. The conditions chosen for these binding assays were shown to result in maximal hormone binding (data not shown).
Second messenger assays
To measure production of cAMP, cells in 24-well plates were washed two times with DMEM/F12 medium (1:1, vol:vol) containing 10 mM HEPES, 50 μg/ml Gentamicin, 0.1% bovine serum albumin and supplemented with 0.5 mM isobutylmethylxanthine and then incubated at 37 C for 15 min in 0.25 ml of the same medium. Triplicate wells were then incubated with hFSH (100 ng/ml), hCG (100 ng/ml) or buffer at 37 C for an additional 30 min after which total cAMP (i.e., cells + medium) was extracted and measured by RIA as described before (36). Under these conditions cAMP accumulation attains a maximal or near maximal level within 30 min of hormone addition and remains elevated for at least 4 hours (30, 37, 38). The concentrations of hormones used were also chosen to elicit maximal cAMP accumulation (30, 37, 38).
To measure the accumulation of inositol phosphates, cells in 12-well plates were washed once with inositol-free DMEM containing 10 mM Hepes, 50 μg/ml gentamicin and 0.1% bovine serum albumin and then placed in 0.5 ml of the same medium containing 4 μCi/ml of [2-3H]myo-inositol (NEN Life Science Products, Boston, MA) during the last 18-h of the post-infection incubation. Cells were then washed two times with DMEM/F12 medium (1:1, vol:vol) containing 10 mM HEPES, 50 μg/ml Gentamicin, 0.1% bovine serum albumin and supplemented with 20 mM LiCl and incubated in 0.5 ml of the same medium at 37 C for 15 min. This was followed by an additional 60-min incubation in the presence of hFSH (500 ng/ml), hCG (500 ng/ml) or buffer. Inositol phosphates were then extracted and quantitated as described (39). These conditions have been shown to result in maximal or near maximal inositol phosphate accumulation in cells expressing the recombinant gonadotropin receptors (30, 37, 38). The concentrations of hormones used were also chosen to elicit maximal inositol phosphate accumulation (30, 37, 38, 40).
Akt phosphorylation assays
Cells in 12-well plates were washed twice with DMEM/F12 medium (1:1, vol:vol) containing 10 mM HEPES, 50 μg/ml gentamicin and 0.1% bovine serum albumin and placed in 1 ml of the same medium during the last 18-h of the post-infection incubation. The medium was then replaced with 0.5 ml of the same medium and the cells were incubated for 30 min in the presence of hFSH (100 ng/ml), hCG (100 ng/ml) or buffer only. At the end of the incubation the cells were placed on ice, quickly washed twice with 1 ml of cold buffer containing 150 mM NaCl and 20 mM HEPES (pH 7.4) and lysed with 35 μl of lysis buffer (150 mM NaCl, 50 mM Tris, 1 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF, pH 7.4) by gentle rocking at 4 C for 30 min. The resulting lysates were clarified by centrifugation and assayed for protein content using the BCA protein assay kit from Bio-Rad Laboratories Inc. (Hercules, CA). Thirty μg of protein from each lysate were then resolved on 12% polyacrylamide gels and transferred electrophoretically to polyvinylidene difluoride membranes (40). This was followed by an overnight incubation of the membranes in a 1:3000 dilution of a phospho-AKT (Ser473) antibody (Cell Signaling Technology Inc, Beverly, MA) followed by a second 1 h incubation with a 1:3000 dilution of a secondary antibody covalently coupled to horseradish peroxidase (Bio-Rad Laboratories Inc.,Hercules, CA). Finally, immune complexes were visualized and quantified using the Super Signal West Femto Maximum Sensitivity detection system (Pierce Chemical Inc, Rockford, IL) and a Kodak digital imaging system (Eastman Kodak Co., Rochester, NY). The effects of hCG and hFSH on the phosphorylation of Akt are transient (41, 42) and our time course experiments (not shown) revealed that a 30-min incubation with hCG or hFSH was optimal for the detection of this response. The concentrations of hormones used for the Akt phosphorylation assays were also chosen to elicit a maximal response (data not shown).
Quantitation of mRNA by Real-time PCR
Two days after infection, medium was aspirated and cells were incubated at 37 C for an additional 48 h with fresh culture medium supplemented with 50 ng/ml testosterone and containing hFSH (100 ng/ml), hCG (100 ng/ml), 8BrcAMP (1 mM) and/or phorbol 12-myristate-13-acetate (PMA, 100 nM). Total RNA was then extracted using Trizol reagent and chloroform followed by isopropanol precipitation. One μg of collected total RNA was reverse-transcribed using Superscript III and random primers (p(dN)6). Aromatase cDNA in the reverse transcription product was quantitated by real time PCR (43) using Sybr Green (Sybr Green PCR Master Mix; Applied Biosystems, Foster City, CA) for amplicon detection and ROX as an internal reference dye. Standard PCR settings (50 C for 2 min, 95 C for 10 min and 40 cycles of 95 C for 15 s and 60 C for 1 min) were used in an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Specific primers (forward, TCCTCCTGATTCGGAATTGTG; reverse, GGCCCGATTCCCAGACA) were used for the reaction. The amount of aromatase mRNA was calculated using ABI Prism 7000 Sequence Detection System software (Applied Biosystems, Foster City, CA). Briefly, a cycle threshold (CT; the first cycle at which a detectable increase in the target amplicon is observed) was obtained for each sample and used to calculate the amount of aromatase mRNA relative to a standard curve (43). GAPDH mRNA was also quantitated in the same samples using specific primers (forward, TGCCAAGTATGATGACATCAAGAAG; reverse, AGCCCAGGATGCCCTTTAGT) and used to correct for variations in RNA content among samples. The 48-hour incubation chosen for the aromatase expression experiments was chosen based on our data (not shown) and those of others (16, 17, 23) who have previously studied this phenomenon.
Statistical methods
To account for experimental variability, within each experiment and endpoint (except for binding data) all values were calculated relative to the value obtained in cells infected with 200 MOI Ad-βgal and treated with FSH (which was taken as 1.0) and are thus shown in the corresponding graphs. Data were then analyzed using the SAS Mixed Procedure (44) and Tukey’s tests were used for comparisons among means. In all cases statistical significance was considered at P < 0.05.
Materials
Purified hCG (CR-127, ~13,000 IU/mg) and purified hFSH (AFP-5720D) were purchased from Dr. A. Parlow of the National Hormone and Pituitary Agency.. Purified recombinant hCG and hFSH were kindly provided by Ares Serono (Randolph, MA). 125I-hCG and 125I-hFSH were prepared as described elsewhere (45). Partially purified hCG (~3,000 IU/mg), purchased from Sigma (St. Louis, MO), and partially purified eFSH, kindly donated by Dr. George Bousfield (Wichita State University, Wichita, KS), were used only for the determination of nonspecific binding (see above). The cAMP antibody and 125I-cAMP used for the cAMP assays were as described before (46). Cell culture medium was obtained from Invitrogen Corp. (Carlsbad, CA). Other supplies and reagents used for granulosa cell extraction and culture were obtained from Sigma-Aldrich Corp. (St Louis, MO), BD Biosciences (San Jose, CA) or Corning (Corning, NY). 8Br-cAMP, PMA and testosterone were obtained from Sigma-Aldrich Corp. (St. Louis, MO). Molecular biology reagents were obtained from Invitrogen Corp. (Carlsbad, CA) or Roche Diagnostics Corp. (Indianapolis, IN). All other chemicals were obtained from commonly used suppliers.
Results
We chose to use adenoviral vectors to induce the expression of recombinant gonadotropin receptors in immature rat granulosa cells because they have shown to mediate high levels of recombinant protein expression in these cells (90% of infected cells) without adversely compromising cellular function (16, 47). Infection with the Ad-hLHR or Ad-hFSHR constructs resulted in the expression of the recombinant receptor as indicated by increased binding of 125I-hCG or 125I-hFSH, respectively (Figures 1 and 5). In addition, the recombinant receptors were functional, as shown by the cAMP, inositol, phospho Akt (Figures 2 and 6), and aromatase (Figures 3 and 7) responses induced upon receptor activation with hCG or hFSH. All of these responses were measured using a saturating hormone concentration (see Materials and Methods) and a single time point. The time points and other experimental conditions selected to measure the different responses are different and they were chosen to provide optimal conditions for measuring each of these individual responses (see Materials and Methods and individual figure legends).
Figure 1. 125I-hFSH and 125I-hCG binding to immature granulosa cells infected with different amounts of Ad-hLHR.
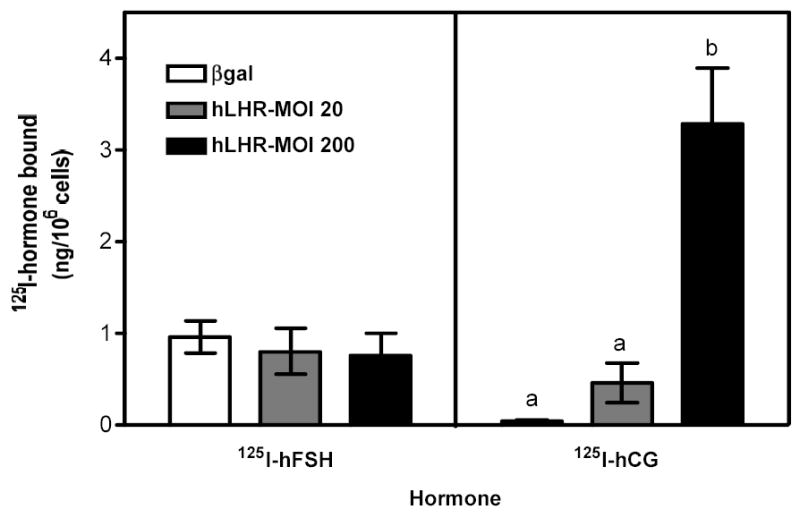
Primary cultures of immature granulosa cells were infected with adenoviral constructs coding for Ad-βgal at 200 MOI (labeled βgal in the figure), a mixture of hLHR at 20 MOI plus Ad-βgal at 180 MOI (labeled hLHR-MOI 20) or Ad-hLHR at 200 MOI (labeled hLHR-MOI 200). The binding of 125I-hFSH (left panel) or 125I-hCG (right panel) were measured 2 days after infection as described in Materials and Methods.
Each bar is the mean ± SEM of 5–10 independent experiments. Means within a panel with different letters (a, b) are significantly different from each other (P<0.05).
Figure 5. 125I-hFSH and 125I-hCG binding to immature granulosa cells after infection with different amounts of Ad-hFSHR.
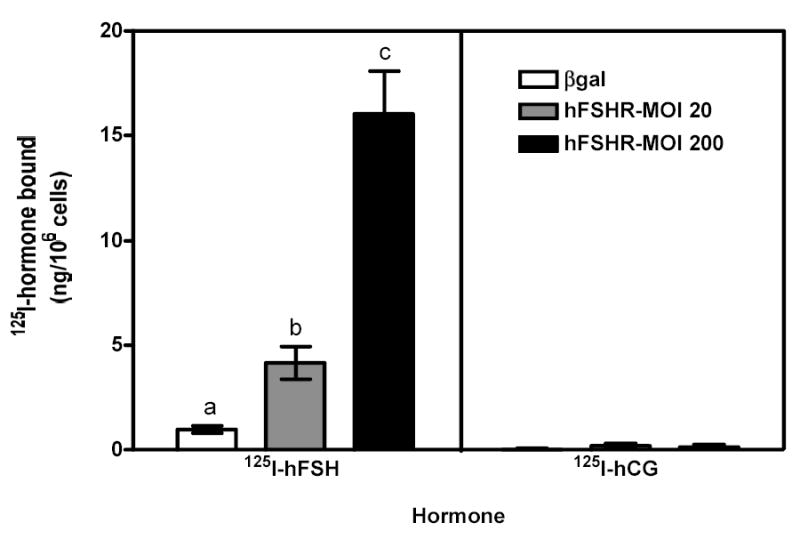
Primary cultures of immature granulosa cells were infected with adenoviral constructs coding for Ad-βgal at 200 MOI (labeled βgal in the figure), a mixture of hFSHR Ad-βgal at 20 MOI plus Ad-βgal at 180 MOI (labeled hFSHR-MOI 20) or Ad-hFSHR at 200 MOI (labeled hFSHR-MOI 200). The binding of 125I-hFSH (left panel) or 125I-hCG (right panel) were measured 2 days after infection as described in Materials and Methods.
Each bar is the mean ± SEM of 5–9 independent experiments. Means within a panel with different letters (a, b, c) are significantly different from each other (P<0.05).
Figure 2 . Human FSH- and hCG-induced cAMP, inositol phosphates and phospho Akt responses in immature granulosa cells infected with different amounts of Ad-hLHR.
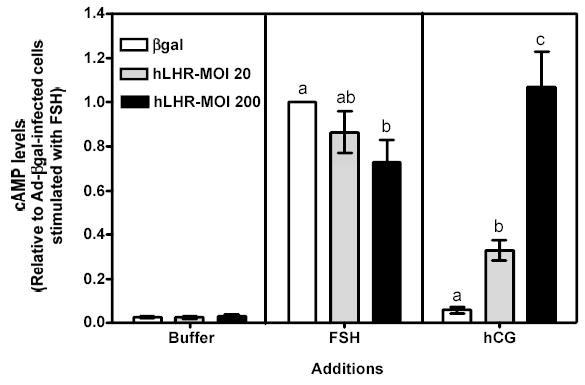
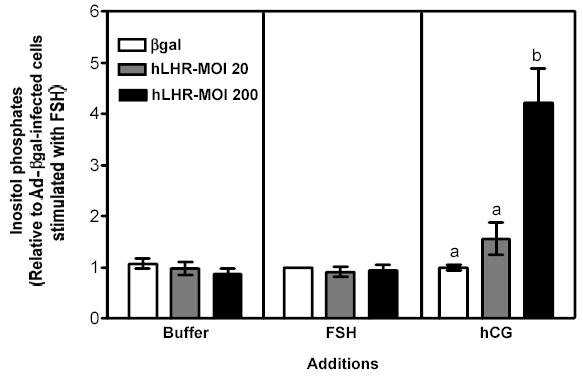
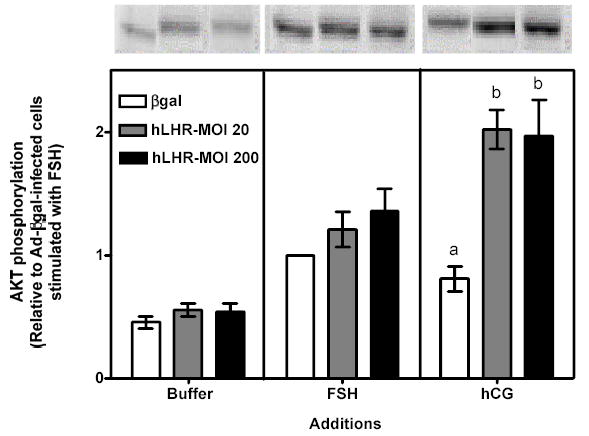
Primary cultures of immature granulosa cells were infected with adenoviral constructs as described in the legend to Figure 1. Two days after infection the cells were incubated with buffer only (left panel), hFSH (middle panel) or hCG (right panel) and the resulting cAMP (panel a), inositol phosphate (panel b) and phospho Akt responses (panel c) were measured as described in Materials and Methods.
For ease of comparison with other figures, and in order to avoid the inherent variability in absolute values associated with the use of primary cultures, all data are expressed relative to the hFSH-induced response of cells infected with βgal (see white bar in the middle panel). Each bar is the mean ± SEM of 4–11 independent experiments. Means within a panel with different letters (a, b, c) are significantly different from each other (P<0.05). The relevant area of representative Western blots documenting the effects of FSH and hCG on Akt phosphorylation are also shown on the top of panel c.
Figure 6 . Human FSH- and hCG-induced cAMP, inositol phosphates and phospho Akt responses in immature granulosa cells infected with different amounts of Ad-hFSHR.
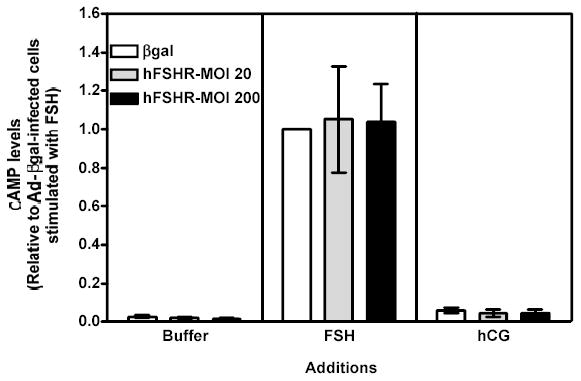
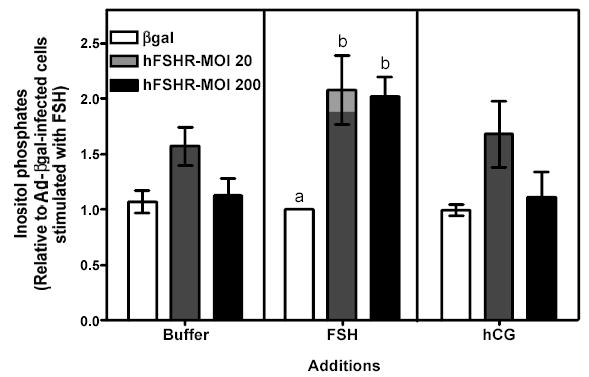
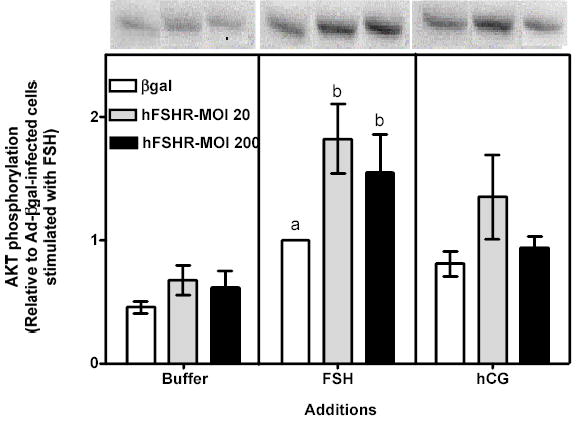
Primary cultures of immature granulosa cells were infected with adenoviral constructs as described in the legend to Figure 5. Two days after infection the cells were incubated with buffer only (left panel), hFSH (middle panel) or hCG (right panel) and the resulting cAMP (panel A), inositol phosphate (panel B), or phospho Akt responses (panel C) were measured as described in Materials and Methods.
For ease of comparison with other figures, and in order to avoid the inherent variability in absolute values associated with the use of primary cultures, all data are expressed relative to the hFSH-induced response of cells infected with βgal (see white bar in the middle panel). Each bar is the mean ± SEM of 5–11 independent experiments. Means within a panel with different letters (a, b) are significantly different from each other (P<0.05). The relevant area of representative Western blots documenting the effects of FSH and hCG on Akt phosphorylation are also shown on the top of panel c.
Figure 3 . Human FSH- and hCG-induced aromatase mRNA in immature granulosa cells infected with different amounts of Ad-hLHR.
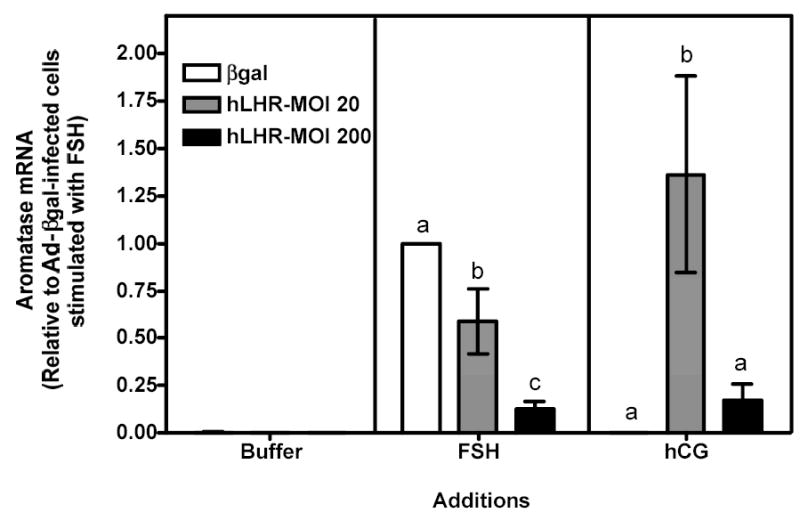
Primary cultures of immature granulosa cells were infected with adenoviral constructs as described in the legend to Figure 1. Two days after infection cells were incubated with buffer only (left panel), hFSH (middle panel) or hCG (right panel). Total RNA was then collected and used to quantify aromatase mRNA using reverse transcription followed by real-time PCR, as described in Materials and Methods.
For ease of comparison with other figures, and in order to avoid the inherent variability in absolute values associated with the use of primary cultures, all data are expressed relative to the hFSH-induced aromatase response of cells infected with βgal (see white bar in the middle panel). The levels of aromatase mRNA in cells incubated with buffer only were often undetectable (see left panel). Each bar is the mean ± SEM of 4–11 independent experiments. Means within a panel with different letters (a, b, c) are significantly different from each other (P<0.05).
Figure 7 . Human FSH- and hCG-induced aromatase mRNA in immature granulosa cells infected with different amounts of Ad-hFSHR.
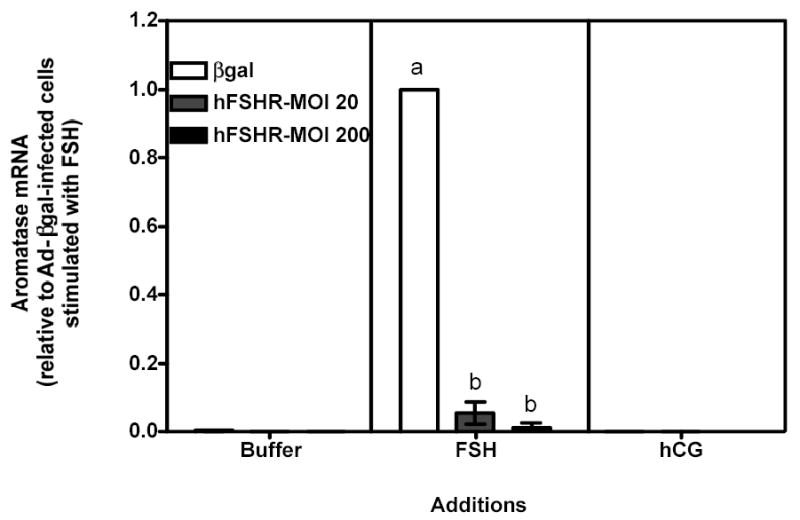
Primary cultures of immature granulosa cells were infected with adenoviral constructs as described in the legend to Figure 5. Two days after infection cells were incubated with buffer only, hFSH (middle panel) or hCG (right panel). Total RNA was then collected and used to quantify aromatase mRNA using reverse transcription followed by real-time PCR, as described in Materials and Methods.
For ease of comparison with other figures, and in order to avoid the inherent variability in absolute values associated with the use of primary cultures, all data are expressed relative to the hFSH-induced aromatase response of cells infected with βgal (see white bar in the middle panel). The levels of aromatase mRNA in cells incubated with buffer only were often undetectable (see left panel). Each bar is the mean ± SEM of 3–5 independent experiments. Means within a panel with different letters (a, b) are significantly different from each other (P<0.05).
As expected, primary cultures of immature rat granulosa cells infected with a control adenovirus coding for β-galactosidase (Ad-βgal) bound 125I-hFSH but did not bind 125I-hCG (Figure 1, white bars in left and right panels). The binding of 125I-hCG dramatically increased in cells infected with 200 MOI Ad-hLHR (Figure 1, right panel) and this increase was associated with increases in the hCG-induced cAMP, inositol phosphate and phospho Akt responses (Figure 2a b and c, right panels). 125I-hCG binding (Figure 1, right panel) in the 20 MOI Ad-hLHR-infected cells was measurable (~0.5 ng/106 cells) but was low compared to binding in 200 MOI Ad-hLHR-infected cells (~3 ng/106 cells), and it was not statistically different from that in the control cells (~0.05 ng/106 cells). However, the clear hCG-induced cAMP and Akt phosphorylation responses observed in the cells infected with 20 MOI Ad-hLHR relative to control cells (Figures 2a and c, right panels) reassured us that the 20 MOI viral load was indeed inducing the expression of functional hLHR. We attribute the different results obtained with these three methods to the higher sensitivity of the cAMP and Akt phosphorylation assays. In interpreting these and all subsequent data it is important to note that the levels of expression of the recombinant LHR in cells infected with the Ad-hLHR are comparable to those attained when the expression of the endogenous LHR is induced by stimulation of these cells with FSH or 8-BrcAMP (35). Immature rat granulosa cells maintained for 3 days under the same culture conditions used here but in the presence of maximally effective concentrations of FSH or 8Br-cAMP bind 1–4 ng of 125I-hCG/106 cells when binding is assayed under the same conditions used here (35).
As mentioned above hCG-induced cAMP and phospho Akt responses were readily measurable in cells infected with 20 or 200 MOI Ad-hLHR (Figures 2a and c, right panels). The hCG-induced cAMP response increased as receptor expression increased (Figure 2a, right panel) but the Akt response was already maximal in cells infected with 20 MOI Ad-hLHR (Figure 2c, right panel). In contrast, only cells expressing the highest density of hLHR (i.e., those infected with 200 MOI Ad-hLHR) responded to hCG with a significant increase in inositol phosphates (Figure 2b, right panel). This is in agreement with results obtained in a number of LHR-transfected cell lines where it has been shown that an inositol phosphate response to hCG stimulation is detectable only at high levels of LHR expression (24). A clear effect of hCG on the induction of aromatase expression was only detected after hCG stimulation of 20 MOI hLHR-infected cells (Figure 3, right panel). The hCG-induced aromatase expression in the cells infected with 200 MOI hLHR was about 85% lower than that detected in the 20 MOI hLHR-infected cells and it was not statistically different from the aromatase response detected in hCG-stimulated control cells (Figure 3, right panel).
To gain insight into the signaling mechanisms involved in the effects of the activated recombinant hLHR on aromatase, we compared the signaling and gene expression responses elicited by hCG in Ad-hLHR-infected cells to those elicited by hFSH in Ad-βgal-infected cells, which express the endogenous FSHR (see white bar on the left panel of Figure 1). As expected, activation of the endogenous FSHR with hFSH induced increases in cAMP levels and Akt phosphorylation (see white bars in the middle panels of Figures 2a and c). Activation of the endogenous FSHR did not result in any detectable accumulation of inositol phosphates, however (see white bar in the middle panel of Figure 2b). The increase in cAMP accumulation was of similar magnitude to that induced by hCG in 200 MOI hLHR-infected cells (compare the white bar in the middle panel with the black bar in the right panel of Figure 2a), and the increase in phospho Akt was less than that induced by hCG in 20 or 200 MOI hLHR-infected cells (compare the white bar in the middle panel with the gray and black bars in the right panel of Figure 2c). In addition, the activation of the endogenous FSHR resulted, as expected, in an increase in aromatase mRNA and this increase was of similar magnitude to the increase in aromatase induced by hCG in the 20 MOI hLHR-infected cells (compare white bar in the middle panel with the gray bar in the right panel in Figure 3). These data clearly support the hypothesis that aromatase can be induced by a common signal or signals (either cAMP alone or a combination of cAMP and phospho Akt) generated by activation of the FSHR and LHR and that a second signal selectively generated by activation of high densities of the LHR antagonizes this induction. Our results (Figure 2b) are also consistent with the hypothesis that the inositol phosphate cascade is this inhibitory signal generated by activation of the LHR when expressed at a high density.
To more directly test the involvement of the activation of the inositol phosphate cascade in the LHR-induced inhibition of aromatase expression, we used Ad-βgal-infected immature granulosa cells to examine the effects of mutational activation of Gαq or pharmacological activation of protein kinase C on aromatase induction by hFSH or 8Br-cAMP (Figure 4). A construct coding for a constitutively active Gαq mutant (GqQ209L) that strongly activates the inositol phosphate cascade (48) was used at a viral load that induced levels of inositol phosphates similar to those induced by activation of the hLHR in 200 MOI hLHR-infected cells (Table 1 and Figure 2b, right panel) and did not affect 125I-hFSH binding or the hFSH-induced cAMP response (Table 1). As shown in Figure 4, 8Br-cAMP induced an increase in aromatase mRNA of similar magnitude to that induced by hFSH and both of these increases were reduced in cells infected with Ad-GqQ209L (Figure 4). In agreement with previous results (26) we also found that pharmacological activation of protein kinase C with PMA diminished the stimulatory effects of hFSH or 8Br-cAMP on aromatase expression (Figure 4). Thus, activation of the inositol phosphate cascade by expression of the constitutively active Gαq or activation of PKC (one of the two branches of this cascade) is sufficient to inhibit the induction of aromatase by hFSH or 8Br-cAMP. These results further indicate that the differential effects of FSH and hCG on the induction of aromatase shown in Figure 3 are mediated by the preferential hLHR-induced activation of the inositol phosphate cascade in cells expressing high densities of hLHR. Furthermore we conclude that this effect is mediated (at least in part) by the activation of PKC because it can be reproduced by pharmacological activation of this kinase.
Figure 4 . Effects of the constitutively active Gαq mutant (GqQ209L) and a pharmacological activator of PKC (PMA) on FSH- and 8BrcAMP-induced aromatase mRNA in immature granulosa cells.
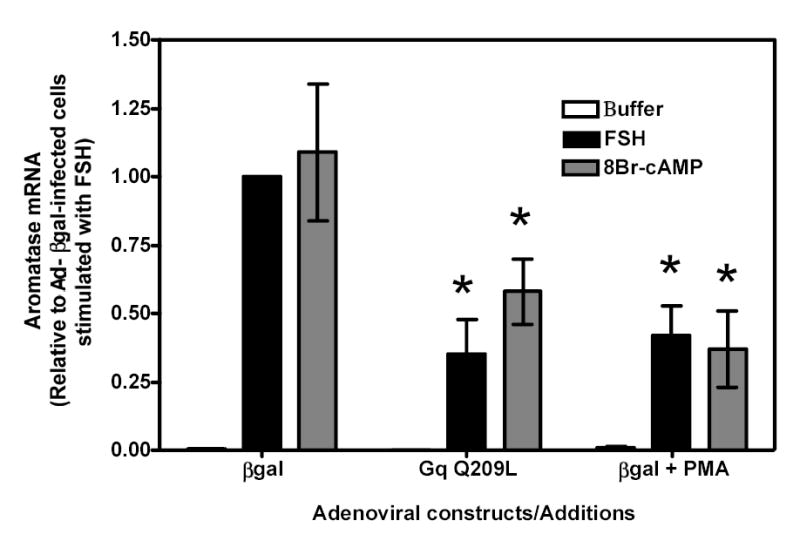
Primary cultures of immature granulosa cells were infected with 200 MOI Ad-βgal (labeled βgal in the figure) or a mixture of 0.5 MOI Ad-GqQ209L and 199.5 MOI (labeled GqQ209L in the figure). Two days later they were incubated with buffer only, hFSH 8BrcAMP, hFSH + PMA or 8Br-cAMP + PMA as shown. Total RNA was then collected and used to quantify aromatase mRNA using reverse transcription followed by real-time PCR, as described in Materials and Methods.
For ease of comparison with other figures, and in order to avoid the inherent variability in absolute values associated with the use of primary cultures, all data are expressed relative to the hFSH-induced aromatase response of cells infected with βgal. The levels of aromatase mRNA in cells incubated with buffer only were often undetectable. Each bar is the mean ± SEM of 5–13 independent experiments. An asterisk indicates a statistically significant difference (P < 0.05) between grouped cells (i.e., those infected with Ad-Gq209L and those infected with Ad-βgal or those infected with Ad-βgal only and incubated with or without PMA)
Table 1.
Effect of infection with Ad-GqQ209L on inositol phosphate, 125I-hFSH binding and cAMP responses in immature granulosa cells
|
Adenoviral construct used for infection |
||
|---|---|---|
| Response measured | βgal | GqQ209L |
| Inositol phosphates accumulation (relative to βgal-infected cells)1 | 1.0 ± 0.0 | 9.8 ± 1.5* |
| 125I-hFSH binding (ng/106 cells) 2 | 1.7 ± 0.1 | 2.0 ± 0.3 |
| FSH-induced cAMP accumulation (relative to βgal-infected cells)2 | 1.0 ± 0.0 | 0.9 ± 0.1 |
Immature granulosa cells were infected with 200 MOI of Ad-βgal or a mixture of 0.5 MOI of Ad-GqQ209L and 199.5 MOI of Ad-βgal. The assays shown were performed 2 days after infection as described in Materials and Methods.
For the inositol phosphate assays the Ad-βgal-infected cells were stimulated with hFSH but the Ad-GqQ209L-infected cells were incubated with buffer only. The asterisk denotes a statistically significant difference (P < 0.05) between the Ad-βgal- and the Ad-GqQ209L-infected cells.
125I-hFSH binding and hFSH-induced cAMP accumulation were measured as described in Materials and Methods. The results are not statistically different (P > 0.1) between Ad-βgal- and Ad-GqQ209L-infected cells.
Because the expression of increasing densities of the hLHR in immature granulosa cells could potentially affect the expression and signaling properties of the endogenous FSHR it is also important to investigate this possibility. This is an important issue because endogenous LHR expression increases as granulosa cells differentiate in vivo (49) and the effects of this increase in LHR density on signaling by the co-expressed endogenous FSHR have not been studied. Interestingly, our data show that expression of increasing densities of the recombinant hLHR affects some aspects of hFSH-induced signaling in these cells. Increased expression of the hLHR had no effect on the binding of 125I-hFSH to the endogenous FSHR (see left panel of Figure 1) or the hFSH-induced phospho Akt response (see middle panel of Figure 2c). There was, however, a ~25% reduction in the hFSH-induced cAMP response in 200 MOI hLHR-infected cells relative to Ad-βgal-infected cells (see middle panel of Fig 2a), as well as a reduction in the hFSH-induced aromatase mRNA in cells infected with 20 MOI hLHR or 200 MOI hLHR (41% and 87%, respectively; see middle panel of Figure 3). Thus, the expression of the recombinant, non activated hLHR directly or indirectly impaired the ability of hFSH to induce aromatase mRNA, and this seemed to involve, at least partially, a decrease in FSHR-stimulated cAMP production.
Since the density of the recombinant hLHR expressed in 200 MOI Ad-hLHR infected cells is 3- to 4-fold higher than the density of the endogenous FSHR (compare the white bar in the left panel and the black bar in the right panel of Figure 1) it was also important to determine if the functional properties of the FSHR change as the density of the FSHR increases. This issue was addressed by analyzing the actions of hFSH and hCG in immature granulosa cells infected with increasing amounts of an adenovirus coding for the hFSHR (Figures 5–7). Infection with 20 or 200 MOI Ad-hFSHR resulted in a ~4- and ~16-fold increase in 125I-hFSH binding, respectively, relative to Ad-βgal-infected cells, which express only the endogenous FSHR (Figure 5, left panel). These increased levels of hFSHR had no effect on the hFSH-induced cAMP response (Figure 6a, middle panel)1 but they elicited a detectable increase in the hFSH-induced inositol phosphate (Figure 6b, middle panel) and they enhanced the hFSH-induced phospho Akt response (Figure 6c, middle panel). Although the magnitude of the phospho Akt response observed in cells expressing the recombinant hFSHR is similar to that mediated by the recombinant hLHR (compare the middle panel of Figure 6c with the right panel of Figure 2c), the magnitude of the inositol phosphate response is lower than that mediated by the recombinant hLHR, especially when receptor density is taken into account. Thus, whereas granulosa cells infected with 200 MOI of Ad-hLHR bound ~3 ng 125I-hCG/106 cells (Figure 1, right panel) and responded to hCG with a ~4-fold increase in inositol phosphates (Figure 2b, right panel), cells infected with 20 or 200 MOI of Ad-hFSHR bound ~4 or ~16 ng 125I-hFSH/106 cells, respectively (Figure 5, left panel) and responded to hFSH with only a ~2-fold increase in inositol phosphate accumulation (Figure 6b, middle panel). Interestingly, at these high levels of hFSHR expression the ability of hFSH to induce aromatase expression was drastically reduced when compared to the cells infected with Ad-βgal (Figure 7, middle panel) in spite of the unchanged hFSH-induced cAMP response (Figure 6a, middle panel) and the enhanced phospho Akt response (Figure 6c, middle panel). Thus, these results are also consistent with the hypothesis that activation of the inositol phosphate cascade (which in this case is mediated by engaging the increased levels of the recombinant hFSHR with hFSH) is responsible for a decrease in the ability of hFSH to induce aromatase expression.
Finally, the effects of Ad-hFSHR infection on endogenous LHR expression and signaling were evaluated in immature rat granulosa cells. As shown in the right panels of Figures 5–7, the increased expression of hFSHR did not affect 125I-hCG binding or hCG-induced cAMP, -inositol phosphate, -phospho AKT or -aromatase mRNA.
Discussion
The identification of the signaling mechanisms underlying the differential effects of FSH and LH on granulosa cells has been considerably limited by the expression of the endogenous LHR and FSHR at different stages of granulosa cell differentiation. Thus, when studying the signaling pathways activated by the endogenous gonadotropin receptors present in granulosa cells a distinction cannot be made between differences in signaling due to receptor type or to changes in the intracellular signaling milieu, i.e., steroidogenic enzymes and signaling proteins, that may occur as a consequence of the differentiation of the granulosa cells.
The observation that high levels of recombinant protein expression can be induced in rat immature granulosa cells by using adenoviral vectors (47) obviated this problem as it allowed for the expression of the recombinant LHR in these cells (16, 17). Here we used adenoviral vectors to express the recombinant hLHR and hFSHR in immature rat granulosa cells and to study the effects of both receptor type and receptor density on signaling pathways and aromatase expression. In agreement with the results of others (16), we conclude that the differential actions of the LH/CG and FSH on granulosa cells are not due to the stage of cell differentiation.
Importantly, our results show that the effects of LHR and FSHR activation on aromatase expression in immature granulosa cells are highly dependent on receptor density and signaling pathways activated. We show that, when expressed at low densities, the activated hLHR can induce aromatase expression to a similar extent as that observed when the endogenous FSHR is activated. When expressed at high densities, however, the activated hLHR is unable to induce aromatase expression. This finding, that the effects of hCG on aromatase expression are dependent on the density of the hLHR expressed is in agreement with previous data showing that a biphasic aromatase response occurs with increasing doses of hCG in cultured differentiated granulosa cells expressing the endogenous LHR (21, 23, 51). In this system low concentrations of hCG stimulate aromatase expression to a similar extent as a maximally effective concentration of FSH whereas higher concentrations of hCG do not stimulate aromatase expression. Our findings underscore the physiological importance of the progressive increase in the density of LHR that occurs during granulosa cell differentiation (49).
The data presented here also show for the first time that with increasing densities of hFSHR the induction of aromatase by the activated FSHR declines substantially (Figure 7). Several studies (15, 52, 53) reported that ovulatory surge doses of FSH have LH-surge like effects in estradiol-primed, hypophysectomized rats or mice, i.e., they induce follicular luteinization and ovulation. Thus, as for the LHR, opposing effects in granulosa cells result from low FSHR densities/low FSH concentrations and high FSHR densities/high FSH concentrations. Since the type of signaling pathways activated by GPCRs may vary as the density of receptors or the amount of agonist used increase (54) we hypothesize that at low signal strengths (i.e., low receptor density and/or agonist concentration) the LHR and FSHR activate a common signaling pathway (or pathways) that is/are stimulatory to the expression of aromatase and that at high signal strengths (i.e., high receptor density and/or agonist concentration) they also activate (similar or distinct) signaling pathways that are inhibitory to the expression of aromatase.
The adenylyl cyclase/cAMP pathway is likely to represent a common pathway activated by the LHR and FSHR that is involved in the induction of aromatase. All investigators agree that the LHR and the FSHR activate this signaling system (6–10) and that addition of cAMP analogs, or the receptor-independent activation of the cAMP signaling system, can induce the expression of aromatase in granulosa cells (1–3, 11, 12, 18, 19, 21–23). The data presented here are consistent with this hypothesis. When exposed to a maximally effective concentration of hFSH, the density of endogenous FSHR present in immature granulosa cells is already high enough to mediate maximal cAMP accumulation and is optimal for hFSH-induced aromatase expression. An increase in the density of FSHR elicited by infection with Ad-hFSHR does not increase the magnitude of the hFSH-induced cAMP response but it substantially reduces the induction of aromatase by hFSH (see Figures 5–7), presumably because of the simultaneous activation of an opposing pathway. When immature granulosa cells are infected with Ad-hLHR the optimal induction of aromatase by hCG occurs at a low density of recombinant hLHR when the magnitude of the hCG-induced cAMP response is lower than that induced by hFSH acting through the endogenous FSHR (see Figures 1–3). As the density of the recombinant hLHR increases the magnitude of the hCG-induced cAMP response also increases but the hCG-induced aromatase expression decreases substantially (see Figures 1–3), again, because of the presumed, simultaneous activation of an opposing pathway.
In view of our results and those of others (17, 41, 42) one must also consider the possibility that the phosphorylation of Akt represents an additional, common pathway activated by the LHR and FSHR that, together with cAMP, is involved in the induction of aromatase. Others have previously shown that injection of hCG results in Akt phosphorylation in the ovaries of rats in diestrus (42) and we have shown here that hCG-increases Akt phosphorylation in immature granulosa cells expressing increasing densities of the recombinant hLHR (Figure 2c). FSH, acting through the endogenous FSHR has also been reported to increase Akt phosphorylation (41, 55). We have confirmed those results here and have also shown that increased expression of the recombinant hFSHR enhances this response (Figure 6c). The involvement of the Akt pathway on the FSH-induced expression of aromatase is supported by the ability of a dominant negative form of Akt to diminish this effect of FSH (17). In addition, Akt overexpression was shown to potentiate the induction of aromatase by FSH, cAMP and a constitutively active form of the LHR (17).
Since there is general agreement that the inositol phosphate cascade is activated when the LHR is expressed at high densities and exposed to high concentrations of hCG (see Figures 1 and 2 and reference 24) and since the activation of this pathway is inhibitory to the cAMP- and FSH-induced aromatase expression (Figure 4 and ref. 26) we propose that the LH/CG-induced activation of the inositol phosphate cascade is responsible for the decrease in aromatase induction observed at high densities of the LHR (Figure 3). This proposal is consistent with the proposed role of PKC activation (a downstream effect of the activation of the inositol phosphate cascade) as a mediator of the changes in aromatase and other ovarian genes that occur at high densities of the LHR (i.e., during luteinization, see refs. 26, 28, 56, 57). It should also be noted that although a relationship between PKC activation and inhibition of aromatase expression has been shown in granulosa cells (26), our results show for the first time a direct association between the activation of the inositol phosphate cascade (induced here by expression of a constitutively active Gαq, see Figure 4) and the suppression of aromatase. This finding is important because although there is agreement about the ability of LH/CG to activate the inositol phosphate cascade, there are conflicting reports on the ability of LH/CG to activate PKC (37, 58). More studies are therefore needed to determine which of the two second messengers generated by activation of the inositol phosphate cascade (inositol phosphates and diacylglycerol) and which effectors of these second messengers are responsible for the inhibition of the cAMP-and FSH-induced aromatase expression. Lastly, as noted here high densities of the FSHR also result in a substantial suppression of the hFSH-induced aromatase expression without a decrease in hFSH-induced cAMP accumulation (Figures 5–7). Interestingly this suppression of aromatase expression is also associated with a modest increase in the ability of hFSH to stimulate the inositol phosphate cascade. Therefore it is possible that this hFSH-induced activation of the inositol phosphate cascade is also responsible for the suppression of aromatase expression that occurs at high levels of FSHR expression.
More definitive proof for the proposed role of the inositol phosphate cascade as an inhibitor of aromatase expression at high densities of the LHR or FSHR can be obtained only by showing that inhibition of the inositol phosphate cascade prevents the suppression of aromatase observed under these conditions. Although pharmacological inhibitors of phospholipase C and PKC are available we have, so far, been unable to use them to further test our hypothesis because of their toxicity. We are now attempting to design gene-silencing approaches to address this important question. We also need to systematically determine if there are other signaling cascades that become activated by gonadotropins acting on a high density of receptors and if they are inhibitory to the cAMP-induced expression of aromatase.
Another novel and interesting finding reported here is that expression of increasing densities of the recombinant hLHR have a negative impact on the cAMP response (Figure 2a) and on aromatase induction (Figure 3) provoked when hFSH activates the endogenous FSHR. This negative effect on hFSH signaling occurs without changes in the density of the endogenous FSHR (Figure 1), is not general because it does not affect the hFSH-induced Akt phosphorylation (Figure 2c), and it is not due to the activation of the recombinant hLHR because it is observed in cells that have not been exposed to hCG. Moreover, since the reduction in the hFSH-induced cAMP response attained in cells expressing high densities of hLHR is small (Figure 2a) it appears unlikely that this decrease would be solely responsible for the large decrease in aromatase induction (Figure 3). This finding needs to be further investigated but several explanations may be considered. First, since we know that GPCRs in general (59, 60) and the hLHR in particular (40) have some intrinsic level of basal activity, it is easy to envision how an increase in the density of the recombinant hLHR may increase the basal activation of signaling pathways that impair the ability of the FSHR to induced aromatase experssion. This is specially true if the signaling pathways involved in the induction of aromatase (such as cAMP and/or phospho-Akt) are already maximally or near maximally activated by engaging the endogenous FSHR. The reduced signaling ability of the endogenous FSHR in cells expressing high hLHR densities may also result from increased dimerization between the endogenous FSHR and the recombinant hLHR. Precedence of this exists with other GPCRs such as the different subtypes of opioid receptors (61, 62). Finally, we (63) and others (64, 65) have reported that the LHR and FSHR can each associate with at least one distinct protein that affects signal transduction. Thus, the increased expression of either receptor could bring increasing amounts of these associating proteins to the plasma membrane where they could affect homologous or heterologous signaling.
In summary, we show that the differential effects of FSH and LH on aromatase in immature granulosa cells are highly dependent on gonadotropin receptor density and on the signaling pathways activated. We propose that aromatase is induced by common signals generated by activation of the FSHR and LHR (either cAMP accumulation alone or a combination of cAMP accumulation and Akt phosphorylation) and that activation of the inositol phosphate cascade in cells expressing a high density of LHR or FSHR antagonizes this induction.
Footnotes
Supported by a grant from the National Institute of Child Health and Human Development (HD-28962).
The lack of effect of increasing hFSHR expression on the hFSH-induced cAMP response was not further investigated. Receptor/G protein-mediated responses are dictated by the levels of receptors and their cognate G proteins as well as their affinity for each other and they can saturate as the levels of receptors increase (50). It is possible that the endogenous FSHR has a high affinity for Gs and that this interaction is already saturated. Alternatively, Gs may not be saturated but additional G proteins that antagonize the actions of Gs on adenylyl cyclase (such as Gi/o) may be engaged by increasing levels of the recombinant hFSHR and these may prevent the expected enhancement in cAMP accumulation.
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in Endocrinology, copyright The Endocrine Society. Cite this article as appearing in 2005 in Endocrinology. This may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article, which is the version of record, can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Richards JS. Hormonal control of gene expression in the ovary. Endocrine Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 2.Richards JS Russell DL, Ochsner S Hsieh M, Doyle KH Falender AE, Lo YK Sharma SC. Novel Signaling Pathways That Control Ovarian Follicular Development, Ovulation, and Luteinization. Rec Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 3.Richards JS. Perspective: The Ovarian Follicle--A Perspective in 2001. Endocrinology. 2001;142:2184–2193. doi: 10.1210/endo.142.6.8223. [DOI] [PubMed] [Google Scholar]
- 4.Pierce JG Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 5.Pierce JG 1988 Gonadotropins: chemistry and biosynthesis. In: Knobil E, Neill JD, Ewing LL, Greenwald GS, Markert CL, Pfaff DW (eds) The physiology of reproduction. Raven Press, New York, pp 1335–1348
- 6.Vassart G Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Simoni M Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology and pathophysiology. Endocrine Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 8.Themmen APN Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocrine Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 9.Dias JA Cohen BD, Lindau-Shepard B Nechamen CA, Peterson AJ Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vit Horm. 2002;64:249–322. doi: 10.1016/s0083-6729(02)64008-7. [DOI] [PubMed] [Google Scholar]
- 10.Segaloff DL Ascoli M. The lutropin/choriogonadotropin (LH/CG) receptor. 4 years later. Endocrine Rev. 1993;14:324–347. doi: 10.1210/edrv-14-3-324. [DOI] [PubMed] [Google Scholar]
- 11.Richards JS. New signaling pathways for hormones and cAMP in endocrine cells. Mol Endocrinol. 2001;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 12.Conti M. Specificity of the Cyclic Adenosine 3′,5′-Monophosphate Signal in Granulosa Cell Function. Biol Reprod. 2002;67:1653–1661. doi: 10.1095/biolreprod.102.004952. [DOI] [PubMed] [Google Scholar]
- 13.Piquette GN LaPolt PS, Oikawa M Hsueh AJW. Regulation of luteinizing hormone receptor messenger ribonucleic acid levels by gonadotropins, growth factors, and gonadotropin releasing hormone in cultured rat granulosa cells. Endocrinology. 1991;128:2449–2456. doi: 10.1210/endo-128-5-2449. [DOI] [PubMed] [Google Scholar]
- 14.Wang C Hsueh AJW, Erickson GF. LH stimulation of estrogen secretion by cultured rat granulosa cells. Mol Cell Endocrinol. 1981;24:17–28. doi: 10.1016/0303-7207(81)90075-7. [DOI] [PubMed] [Google Scholar]
- 15.Tapanainen JS Lapolt PS, Perlas E Hsueh AJW. Induction of ovarian follicle luteinization by recombinant follicle-stimulating hormone. Endocrinology. 1993;133:2875–2880. doi: 10.1210/endo.133.6.8243314. [DOI] [PubMed] [Google Scholar]
- 16.Bebia Z Somers JP, Liu G Ihrig L, Shenker A Zeleznik AJ. Adenovirus-Directed Expression of Functional Luteinizing Hormone (LH) Receptors in Undifferentiated Rat Granulosa Cells: Evidence for Differential Signaling through Follicle-Stimulating Hormone and LH Receptors. Endocrinology. 2001;142:2252–2259. doi: 10.1210/endo.142.6.8017. [DOI] [PubMed] [Google Scholar]
- 17.Zeleznik AJ Saxena D, Little-Ihrig L. Protein Kinase B Is Obligatory for Follicle-Stimulating Hormone-Induced Granulosa Cell Differentiation. Endocrinology. 2003;144:3985–3994. doi: 10.1210/en.2003-0293. [DOI] [PubMed] [Google Scholar]
- 18.Simpson ER Clyne C, Rubin G Boon WC, Robertson K Britt K, Speed C Jones M. Aromatase, a brief overview. Annual Review of Physiology. 2002;64:93–127. doi: 10.1146/annurev.physiol.64.081601.142703. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Robayna IJ Alliston TN, Buse P Firestone GL, Richards JS. Functional and Subcellular Changes in the A-Kinase-Signaling Pathway: Relation to Aromatase and Sgk Expression during the Transition of Granulosa Cells to Luteal Cells. Mol Endocrinol. 1999;13:1318–1337. doi: 10.1210/mend.13.8.0334. [DOI] [PubMed] [Google Scholar]
- 20.Segaloff DL Wang H, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular developmen and luteinization. Mol Endocrinol. 1990;4:1856–1865. doi: 10.1210/mend-4-12-1856. [DOI] [PubMed] [Google Scholar]
- 21.Hickey GJ Krasnow JS, Beattie WG Richards JS. Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3′,5′-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase and 5′ genomic. DNA Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick SL Richards JS. Cis-acting elements of the rat aromatase promoter required for cyclic adenosine 3′,5′-monophosphate induction in ovarian granulosa cells and constitutive expression in R2C cells. Mol Endocrinol. 1993;7:341–354. doi: 10.1210/mend.7.3.8387157. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick SL Richards JS. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology. 1991;129:1452–1462. doi: 10.1210/endo-129-3-1452. [DOI] [PubMed] [Google Scholar]
- 24.Ascoli M Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor. A 2002 perspective Endocrine Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara O Knecht M, Catt KJ. Inhibition of gonadotropin-induced granulosa cell differentiation by activation of protein kinase C. Proc Natl Acad Sci (USA) 1985;82:8518–8522. doi: 10.1073/pnas.82.24.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzpatrick SL Carlone DL, Robker RL Richards JS. Expression of aromatase in the ovary: down-regulation of mRNA by the ovulatory luteinizing hormone surge. Steroids. 1997;62:197–206. doi: 10.1016/s0039-128x(96)00181-x. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski t Akinola L, Poutanen M Vihko R, Vihko P. Growth factors and phorbol-12-myristate-13-acetate modulate the follicle-stimulating hormone- and cyclic adenosine-3′5′-monosphosphate-dependent regulation of 17beta-hydroxysteroid dehydrogenase type 1 expression in rat granulosa cells. Mol Cell Endocrinol. 1997;31:47–56. doi: 10.1016/s0303-7207(97)00213-x. [DOI] [PubMed] [Google Scholar]
- 28.Jo M Curry TE., Jr Regulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biol Reprod. 2004;71:1796–1806. doi: 10.1095/biolreprod.104.031823. [DOI] [PubMed] [Google Scholar]
- 29.Min L Ascoli M. Effect of activating and inactivating mutations on the phosphorylation and trafficking of the human lutropin/choriogonadotropin receptor. Mol Endocrinol. 2000;14:1797–1810. doi: 10.1210/mend.14.11.0555. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy H Kishi H, Shi M Galet C, Bhaskaran RS Hirakawa T, Ascoli M. Post-endocytotic trafficking of the FSH/FSH receptor complex. Mol Endocrinol. 2003;17:2162–2176. doi: 10.1210/me.2003-0118. [DOI] [PubMed] [Google Scholar]
- 31.Graziano MP Gilman AG. Synthesis in Escherichia coli of GTPase-deficient mutants of Gsα. J Biol Chem. 1989;264:15475–15482. [PubMed] [Google Scholar]
- 32.Liri T Farfel Z, Bourne HR. G-protein diseases furnish a model for the turn-on switch. Nature. 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 33.Farfel Z Bourne HR, ri T. The expanding spectrum of G protein diseases. New Engl J Med. 1999;340:1012–1020. doi: 10.1056/NEJM199904013401306. [DOI] [PubMed] [Google Scholar]
- 34.Yoshino M Mizutani T, Yamada K Tsuchiya M, Minegishi T Yazawa T, Kawata H Skiguchi T, Kajitani T Miyamoto K. Early growth response gene-1 regulates the expression of the rat luteinizing hormone receptor gene. Biol Reprod. 2002;66:1813–1819. doi: 10.1095/biolreprod66.6.1813. [DOI] [PubMed] [Google Scholar]
- 35.Shi H Segaloff DL. A role for increased lutropin/choriogonadotropin receptor (LHR) gene transcription in the follitropin-stimulated induction of the LHR in granulosa cells. Mol Endocrinol. 1995;9:734–744. doi: 10.1210/mend.9.6.8592519. [DOI] [PubMed] [Google Scholar]
- 36.Steiner AL Parker CW, Kipnis DM. Radioimmunoassay for cyclic nucleotides. J Biol Chem. 1972;247:1106–1113. [PubMed] [Google Scholar]
- 37.Hipkin RW Wang Z, Ascoli M. Human chorionic gonadotropin- and phorbol ester stimulated phosphorylation of the LH/CG receptor maps to serines 635, 639, 645 and 652 in the C-terminal cytoplasmic tail. Mol Endocrinol. 1995;9:151–158. doi: 10.1210/mend.9.2.7776965. [DOI] [PubMed] [Google Scholar]
- 38.Hipkin RW Liu X, Ascoli M. Truncation of the C-terminal tail of the follitropin (FSH) receptor does not impair the agonist- or phorbol ester-induced receptor phosphorylation and uncoupling. J Biol Chem. 1995;270:26683–26689. doi: 10.1074/jbc.270.44.26683. [DOI] [PubMed] [Google Scholar]
- 39.Ascoli M Pignataro OP, Segaloff DL. The inositol phosphate/diacylglycerol pathway in MA-10 Leydig tumor cells. Activation by arginine vasopressin and lack of effect of epidermal growth factor and human choriogonadotropin. J Biol Chem. 1989;264:6674–6681. [PubMed] [Google Scholar]
- 40.Hirakawa T Galet C, Ascoli M. MA-10 cells transfected with the human lutropin/choriogonadotropin receptor (hLHR). A novel experimental paradigm to study the functional properties of the hLHR. Endocrinology. 2002;143:1026–1035. doi: 10.1210/endo.143.3.8702. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Robayna IJ Falender AE, Ochsner S Firestone GL, Richards JS. Follicle-Stimulating Hormone (FSH) Stimulates Phosphorylation and Activation of Protein Kinase B (PKB/Akt) and Serum and Glucocorticoid-Induced Kinase (Sgk): Evidence for A Kinase-Independent Signaling by FSH in Granulosa Cells. Mol Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- 42.Carvalho CRO Carvalheira JBC, Lima MHM Zimmerman SF, Caperuto LC Amanso A, Gasparetti AL Meneghetti V, Zimmerman LF Velloso LA, Saad MJA. Novel Signal Transduction Pathway for Luteinizing Hormone and Its Interaction with Insulin: Activation of Janus Kinase/Signal Transducer and Activator of Transcription and Phosphoinositol 3-Kinase/Akt Pathways. Endocrinology. 2003;144:638–647. doi: 10.1210/en.2002-220706. [DOI] [PubMed] [Google Scholar]
- 43.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 44.Institute S 1995 Introduction to the MIXED procedure. SAS Institute, INC, Cary, NC
- 45.Ascoli M Puett D. Gonadotropin binding and stimulation of steroidogenesis in Leydig tumor cells. Proc Natl Acad Sci (USA) 1978;75:99–102. doi: 10.1073/pnas.75.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segaloff DL Ascoli M. Removal of surface-bound human choriogonadotropin results in cessation of hormonal responses in cultured Leydig tumor cells. J Biol Chem. 1981;256:11420–11423. [PubMed] [Google Scholar]
- 47.Somers JP DeLoia JA, Zeleznik AJ. Adenovirus-Directed Expression of a Nonphosphorylatable Mutant of CREB (cAMP Response Element-Binding Protein) Adversely Affects the Survival, but Not the Differentiation, of Rat Granulosa Cells. Mol Endocrinol. 1999;13:1364–1372. doi: 10.1210/mend.13.8.0329. [DOI] [PubMed] [Google Scholar]
- 48.De Vivo M Chen J, Codina J Iyengar R. Enhanced phospholipase C stimulation and transformation in NIH-3T3 cells expressing Q209LGq-alpha subunits. J Biol Chem. 1992;267:18263–18266. [PubMed] [Google Scholar]
- 49.Uilenbroek JT Richards JS. Ovarian follicular development during the rat estrous cycle: gonadotropin receptors and follicular responsiveness. Biol Reprod. 1979;20:1159–1165. doi: 10.1095/biolreprod20.5.1159. [DOI] [PubMed] [Google Scholar]
- 50.Whaley BS Yuan N, Birnbaumer L Clark RB, Barber R. Differential expression of the β–adrenergic receptor modifies agonist stimulation of adenylyl cyclase: a quantitative evaluation. Mol Pharmacol. 1994;45:481–489. [PubMed] [Google Scholar]
- 51.Yong EL Hillier SG, Turner M Baird DT, Ng SC Bongso A, Ratnam SS. Differential regulation of cholesterol side-chain cleavage (P450scc) and aromatase (P450arom) enzyme mRNA expression by gonadotrophins and cyclic AMP in human granulosa cells. J Mol Endocrinol. 1994;12:239–249. doi: 10.1677/jme.0.0120239. [DOI] [PubMed] [Google Scholar]
- 52.Galway AB, Lapolt PS, Tsafriri A, Dargan CM, Boime I, Hsueh AJW 1990 Recombinant follicle-stimulating hormone induces ovulation and tissue plasminogen activator expression in hypophysectomized rats. Endocrinology 127 [DOI] [PubMed]
- 53.Wang XN Greenwald GS. Human chorionic gonadotropin or human recombinant follicle-stimulating hormone (FSH)-induced ovulation and subsequent fertilization and early embryo development in hypophysectomized FSH-primed rates. Endocrinology. 1993;132:2009–1016. doi: 10.1210/endo.132.5.8477652. [DOI] [PubMed] [Google Scholar]
- 54.Zhu X Gilbert S, Birnbaumer M Birnbaumer L. Dual signaling potential is common among Gs-coupled receptors and dependent on receptor density. Mol Pharmacol. 1994;46:460–469. [PubMed] [Google Scholar]
- 55.Cunningham MA Zhu Q, Unterman TG Hammond JM. Follicle-Stimulating Hormone Promotes Nuclear Exclusion of the Forkhead Transcription Factor FoxO1a via Phosphatidylinositol 3-Kinase in Porcine Granulosa Cells. Endocrinology. 2003;144:5585–5594. doi: 10.1210/en.2003-0678. [DOI] [PubMed] [Google Scholar]
- 56.Morris J Richards J. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology. 1995;136:1549–1558. doi: 10.1210/endo.136.4.7895665. [DOI] [PubMed] [Google Scholar]
- 57.Morris J Richards J. Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology. 1993;133:770–779. doi: 10.1210/endo.133.2.8393774. [DOI] [PubMed] [Google Scholar]
- 58.Salvador LM Maizels E, Hales DB Miyamoto E, Yamamoto H Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143:2986–2994. doi: 10.1210/endo.143.8.8976. [DOI] [PubMed] [Google Scholar]
- 59.Gether U. Uncovering molecular mechanisms involved in activation of G protein- coupled receptors. Endocrine Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- 60.Kenakin T. Principles: Receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- 62.Terrillon S Bouvier M. Roles of G protein-coupled receptor dimerization. EMBO Reports. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirakawa T Galet C, Kishi M Ascoli M. GIPC binds to the human lutropin receptor (LHR) through an unusual PDZ domain binding motif and it regulates the sorting of the internalized human choriogonadotropin (hCG) and the density of cell surface LHR. J Biol Chem. 2003;278:49348–49357. doi: 10.1074/jbc.M306557200. [DOI] [PubMed] [Google Scholar]
- 64.Nechamen CA Thomas RM, Cohen BD Acevedo G, Poulikakos PI Testa JR, Dias JA. Human Follicle-Stimulating Hormone (FSH) Receptor Interacts with the Adaptor Protein APPL1 in HEK 293 Cells: Potential Involvement of the PI3K Pathway in FSH Signaling. Biol Reprod. 2004;71:629–636. doi: 10.1095/biolreprod.103.025833. [DOI] [PubMed] [Google Scholar]
- 65.Cohen BD Nechamen CA, Dias JA. Human follitropin receptor (FSHR) interacts with the adapter protein 13-3-3τ. Mol Cell Endocrinol. 2004;220:1–7. doi: 10.1016/j.mce.2004.04.012. [DOI] [PubMed] [Google Scholar]


