Abstract
Background
X inactivation in mammals results in the transcriptional silencing of an X chromosome in females, and this inactive X acquires many of the epigenetic features of silent chromatin. However, not all genes on the inactive X are silenced, and we have examined the TIMP1 gene, which has variable inactivation amongst females. This has allowed us to examine the features permitting expression from the otherwise silent X by comparing inactive X chromosomes with and without TIMP1 expression.
Results
Expression was generally correlated with euchromatic chromatin features, including DNA hypomethylation, nuclease sensitivity, acetylation of histone H3 and H4 and hypomethylation of H3 at lysines 9 and 27. Demethylation of the TIMP1 gene by 5-azacytidine was able to induce expression from the inactive X chromosome in somatic cell hybrids, and this expression was also accompanied by features of active chromatin. Acetylated histone H3 continued to be observed even when expression was lost in cells that naturally expressed TIMP1; while acetylation was lost upon TIMP1 silencing in cells where expression from the inactive X had been induced by demethylation. Thus ongoing acetylation of inactive X chromosomes does not seem to be simply a 'memory' of expression.
Conclusion
We propose that acetylation of H3 is an epigenetic mark that predisposes to TIMP1 expression from the inactive X chromosome in some females.
Background
Studies have shown considerable individual variability in the level of expression of genes (e.g. [1,2]). In general, however, humans cannot tolerate imbalances for expression of substantial numbers of genes, as demonstrated by the lethality of the majority of chromosomal aneuploidies. Aneuploidy for the sex chromosomes is better tolerated, being observed in approximately 1/500 births [3], presumably because all but one X chromosome is inactivated. X chromosome inactivation ensures the dosage equivalence of X-linked genes between females who have two X chromosomes and males who have a single X chromosome and the sex-determining Y chromosome [4]. However, more than 15% of human X-linked genes escape inactivation, being expressed from both the active and inactive X chromosome [5]. While such an escape from inactivation may maintain dosage equivalence for X-linked genes with Y homologs, the majority of human genes that escape inactivation no longer have functional Y equivalents, and thus may show relative overexpression in females (reviewed in [6]). Substantially fewer genes have been shown to escape inactivation in mice. Although this species difference in expression could reflect less extensive murine expression surveys, a reduced number of genes escaping inactivation is supported by the less drastic phenotype caused by monosomy of the X chromosome in mice (reviewed in [7]). In humans, the over or under-expression of genes that escape inactivation is a major contributor to the phenotypes associated with X chromosome aneuploidies, but may also contribute to expression differences between chromosomally normal males and females (e.g. [8]).
The study of genes that escape X inactivation can provide insight into such phenotypes, as well as contributing to our understanding of epigenetic silencing mechanisms. Inactivation occurs early in mammalian development, and the stable silencing of the X chromosome involves the acquisition of many features of heterochromatin. It is not known if escape from inactivation is a resistance to the initial silencing event, or rather reflects a high frequency of reactivation of an initially silenced gene, as data appear to support both possibilities. Many genes that escape inactivation in humans are clustered together, which may be indicative of regions that are resistant to the initial signal (e.g. [9]). However, analysis of Smcx, one of the few mouse genes expressed from the murine inactive X, has shown reactivation of the gene during early development [10]. Surprisingly, recent results have shown that Smcx has a histone modification pattern suggested to demarcate biallelically rather than monoallelically-expressed (imprinted and other X-linked) genes [11], suggesting that the gene is committed to escape inactivation prior to undergoing inactivation.
In addition to the genes that are subject to, or escape from, inactivation, there are some human genes that show heterogeneous X chromosome inactivation, being expressed from the inactive X in some females, but silenced on the inactive X in others [5,12,13]. Such genes provide an opportunity to study the same region when silent or active on an inactive X chromosome; and can thus provide insights into the features allowing expression from the inactive X chromosome. The human inactive X chromosome maintains its silent status when isolated in a mouse/human somatic cell hybrid, providing a model system to study the inactive X chromosome apart from its active counterpart. The largest survey of gene expression from the inactive X chromosome [5] analysed expression in a panel of nine inactive-X containing hybrids. That study defined heterogeneous inactivation as expression in three to six of the nine hybrids, which was observed for 60 of the 624 X-linked genes analysed. This variable expression is not restricted to the hybrid system, but has also been demonstrated in cells from females [5,12,13].
We now report the further characterization of one of these genes, the X-linked tissue inhibitor of metalloproteinases, TIMP1, located in Xp11.23. Our previous studies have demonstrated that TIMP1 is variably expressed from the inactive X in both somatic cell hybrids and human females. When TIMP1 is expressed from the inactive X, flanking genes (including ARAF1 and ELK1 which lie ~20 and 55 kb from TIMP1, respectively) are not expressed, suggesting that expression is being controlled in a gene-specific rather than regional fashion [12]. Quantitative RNase protection assays showed substantial variability in expression levels from the active X, precluding using expression levels to determine inactive X expression in females. Further studies in hybrids demonstrated that TIMP1-expressing clones were unstable and that methylation does not appear to be the principle controlling feature allowing variable expression of TIMP1 from the inactive X chromosome [14]. In this study we report the analysis of other features characteristic of an inactive X to determine which features might predispose TIMP1 to expression from the inactive X chromosome in a subset of females.
The inactive X acquires many of the general features of heterochromatin (reviewed in [15]), and we have now examined replication timing, nuclease sensitivity, and histone modifications for TIMP1. Late replication of the inactive X at the chromosome level is observed after Giemsa staining following bromodeoxyuridine incorporation [16]. Regions such as the distal and proximal short arm that contain a large proportion of genes that escape inactivation are not delayed in their replication, supporting a regional basis to escape from inactivation. Replication of individual X-linked genes has been analysed by fluorescent in situ hybridization (FISH) or amplification of BuDR incorporated DNA after flow cytometry. Although correspondence is not complete between different techniques [17], such methods have generally shown that genes that escape inactivation are early replicating (reviewed in [18]). DNase sensitivity is a general feature of active chromatin, and promoters of genes subject to inactivation have been seen to be less available for digestion by nucleases (e.g. [19]). Modifications to the histones associated with the inactive X are reflective of both general heterochromatic changes and ones specific to the facultative heterochromatin of the inactive X. Using antibodies to acetylated histones the inactive X chromosome stains very palely [20], while antibodies to histone H3 methylated at lysine (K) 9 (H3mK9) are generally associated with heterochromatin and those to methylated lysine 27 (H3mK27) specifically mark the inactive X chromosome [21,22]. Chromatin immunoprecipitation (ChIP) has revealed that genes subject to inactivation show limited acetylation and elevated histone methylation (H3mK9/27) at their promoters relative to genes escaping inactivation [23,24].
Results
Replication timing of TIMP1 in human cells
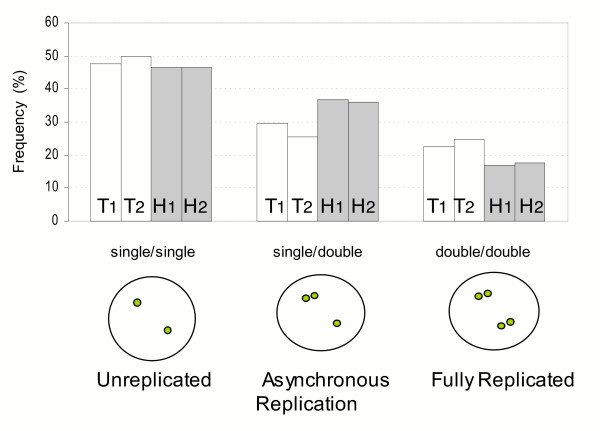
To evaluate the influence of replication timing upon expression of TIMP1, cell lines from a female with and a female without TIMP1 expression from the inactive X [12] were analysed by DNA FISH using probes for the TIMP1 and HPRT loci. As shown in the schematic of Figure 1, cells that have not replicated either X chromosome will show two single signals, and almost one half of the cells were of this type. Of the cells with at least one double signal, indicating replication of the locus, considerable asynchrony was observed for the HPRT locus, as 37% of all cells analysed showed single-double (SD) signals and 17% showed the double-double (DD) signals. The TIMP1 locus generally showed lower asynchrony of replication than HPRT (28% of cells were S/D vs 37% for HPRT, P < 0.0001). The cell line with TIMP1 expression from the inactive X (cell line 2 – HSC593) showed a small trend towards having a lower percentage of cells that replicated asynchronously than the GM07059 cell line which does not have TIMP1 expression from the inactive X (25% vs 29%); however this difference was not significant (P = 0.17). The observed shift in replication timing, while not statistically significant, may be a contributing factor in the expression of TIMP1 from the inactive X. However, it is likely that additional changes are also involved, as TIMP1 is within 20 kb of the ARAF1 gene and 55 kb from ELK1 (see Figure 2A), and thus at least one of these genes is likely to share a replication domain with TIMP1 despite remaining silent when TIMP1 is expressed [12].
Figure 1.
Replication asynchrony for the TIMP1 (T – white bars) and HPRT (H – grey bars) genes assessed by FISH. The bars show the frequency of nuclei exhibiting unreplicated (single/single); asynchronously replicated (single/double) and completely replicated (double/double) signals as shown in the schematic below. The TIMP1 and HPRT probes were individually hybridized to interphase nuclei of the same preparations. Two human female lymphoblast cell lines were examined for the approximate degree of replication asynchrony, one that inactivated TIMP1 (1 – GM07059) and one that expressed TIMP1 from the Xi (2 – HSC593). For TIMP1, 313 cells were counted from 3 separate cell harvests for cell line 1 (T1) and 217 cells for cell line 2 (T2) from two separate harvests. 144 cells were examined for HPRT from 2 slides of one harvest for cell line 1 (H1) and 97 cells from a single harvest for cell line 2 (H2). Between different cell harvests of the same cell line the maximal difference in frequencies of nuclei in each of the three replication classes was 3%.
Figure 2.
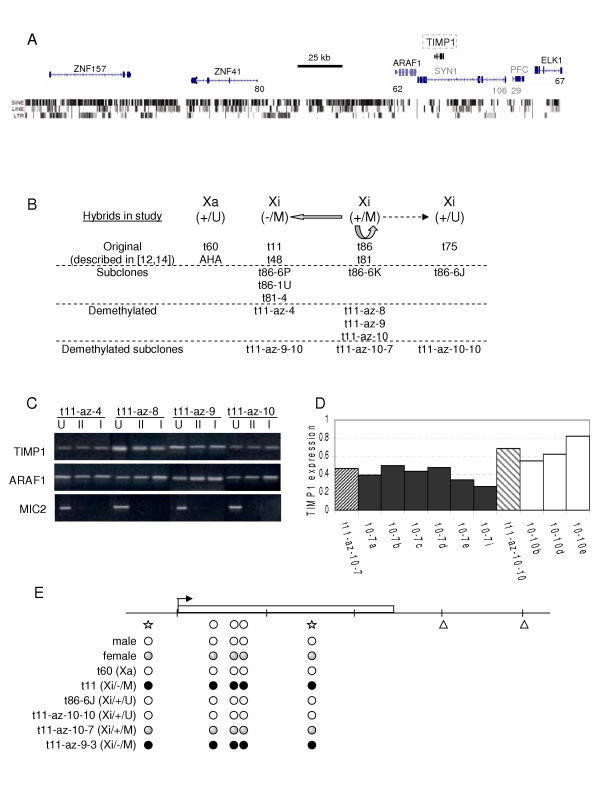
Methylation of TIMP1. A. The genes in the region surrounding TIMP1 are shown with the gene name written above the line diagram for the gene (with vertical lines representing exons and small arrows showing transcriptional orientation). The number listed on the line below at the 5' end of the genes indicates the CpG island density. The TIMP1 and ZNF147 genes do not have enough CpG sites to qualify as a CpG island so there is no number listed. The SYN1, and PFC genes are listed in grey because these genes (which are expressed in a tissue-specific manner) were not examined in this study. The presence of repeat elements (SINE, LINE and LTR) is indicated by the vertical lines below the genes. This figure is based on data generated by the UCSC browser http://www.genome.ucsc.edu/ hg17 of NCBI Build 35. B. Somatic cell hybrids analysed for TIMP1 activity. In addition to the stable active X chromosomes that express TIMP1 and are unmethylated at the 5' end of the gene (Xa/+/U) there are several categories of inactive X chromosomes. Most inactive X chromosomes previously analysed do not express TIMP1 and are methylated (Xi/-/M). Other inactive X chromosomes express TIMP1 and are unmethylated (Xi/+/U), while an intermediary class of hybrids showed both DNA methylation and lower expression levels (Xi/+/M). Subcloning of these latter cells showed that they were unstable, giving rise to additional methylated expressing clones as well as methylated silent clones and expressing unmethylated clones. The arrows show approximate proportion of cells of each class derived from subcloning. Subclones further characterized are listed below, as are clones derived by 5-azacytidine treatment and their subclones (see Tables 1 and 2). C. DNA methylation of clones from four of the demethylated clones listed in Table 1. DNA from each clone was digested with EcoRI alone (U), EcoRI plus HpaII (II) or EcoRI plus HhaI (I). Primers for the 5' end of TIMP1 and ARAF1 that flank HpaII or HhaI methylation-sensitive restriction enzyme sites were used to assess methylation, while amplification of MIC2, which is unmethylated on both active and inactive X chromosomes, served as a control for complete digestion with the methylation-sensitive enzymes. D. Comparison of expression levels in subclones of two 'sibling' subclones of t11-az-10 differing in methylation states. The t11-az-10-7 and t11-az-10-10 clones are striped, with their subclones shown to their right. Methylation (dark fill) was observed for t11-az-10-7 and its subclones while t11-az-10-10 and its subclones were unmethylated (unfilled). All subclones continued to express both TIMP1 and ARAF1 as assayed by RT-PCR. Despite the relative stability of the methylated TIMP1+ culture, the TIMP1 expression level was significantly lower in the methylated cultures (p < 0.01). E. Methylation analysis by bisulphite treatment. The 5' end of the TIMP1 gene was sequenced after bisulfite conversion, which changes unmethylated Cs to Us but leaves methylated Cs unchanged. Therefore, the presence of a C indicates that the CpG was methylated. The following CpG sites were analyzed: HhaI sites (stars) at -3 and +31; and three other sites not analyzed by methylation-sensitive enzymes (circles) at +11, +17, +20. The HpaII sites (triangles) at +61 and +81 were used in methylation-sensitive assays but were not reliably analysed by bisulphite sequencing. The open circles indicate unmethylated CpGs whereas the filled circles represent methylated CpGs. The shaded circles designate that both converted and unconverted bases were seen after sequencing, indicating that both methylated and unmethylated CpGs were present. Cell lines are listed, the male cells were GM7057 and the female cells were GM7059. t11-az-9-3 is a TIMP1- subclone of t11-az-9.
DNA methylation of TIMP1 in somatic cell hybrids
Analysis of alterations to the inactive X chromosome is complicated by the presence of the active X chromosome in female cells, so we have analysed features of inactive X chromosomes isolated in mouse/human somatic cell hybrids. We have previously described the characterization of DNA methylation status and expression levels in a number of these hybrids [14] and Figure 2B presents an outline of the hybrids analysed in this study. In addition to hybrids retaining the active X chromosome (t60-12 (t60) and AHA11aB1 (AHA)), in which TIMP1 was unmethylated and expressed, three classes of inactive X-containing hybrids were previously described. Those that were methylated and did not have TIMP1 expression (Xi/-/M – t11-4Aaz-5 (t11) and t48-1a-1Daz4a (t48)) or expressed TIMP1 and were unmethylated (Xi/+/U – t75-2maz34-1a (t75)) were stable, with no gain or loss of expression. In contrast, the two hybrids that expressed TIMP1 but were methylated at the TIMP1 promoter (Xi/+/M – t86-B1maz1b-3a (t86) and t81-az1D (t81)) demonstrated instability as subclones could be either methylated and expressing (Xi/+/M) or methylated and silent (Xi/-/M) as well as occasionally unmethylated and expressing (Xi/+/U). Consistently the Xi/-/M and Xi/+/U subclones tended to be stable while Xi/+/M subclones were unstable. As the methylated-expressing clones (Xi/+/M) are unstable and give rise to a mixed population of methylated silent and unmethylated expressing clones, these clones do not indicate if loss of methylation follows, or predisposes to, expression.
To further test the role of methylation in regulating expression in the TIMP1 region, an inactive X-containing hybrid that did not express TIMP1 (t11) was treated with 5-azacytidine, which is known to induce reactivation of X-linked genes in somatic cell hybrids. As there is not a selectable marker for TIMP1 reactivation, clones were initially selected for HPRT reactivation to ensure that the clones had been demethylated and potentially increase the frequency of reactivation, as co-reactivation of different X-linked genes has been observed after 5-azacytidine treatment [25]. 15 HPRT+ clones were selected in HAT media, and examined by RT-PCR for TIMP1 as well as flanking gene expression (Table 1). After three passages, six clones showed expression of TIMP1. A number of flanking genes were also observed to reactivate with 5-azacytidine treatment; with one clone expressing ARAF1, five expressing ELK1, three expressing ZNF41 and eight expressing ZNF157. The single ARAF1-expressing clone, and all five ELK1-expressing clones, were observed in TIMP1+ clones, and all three ZNF41 positive clones were ZNF157 positive. The TIMP1 and ZNF157 genes lack a CpG island (see Figure 2A), and TIMP1 and ZNF157 showed the highest (6/15 and 8/15) reactivation frequencies after 5-azacytidine treatment. However, ARAF1 showed the lowest reactivation frequency (1/15) despite having a smaller CpG island than either ELK1 or ZNF41, so reactivation frequency was not simply a reflection of CpG density.
Table 1.
Expression of TIMP1 and surrounding genes following 5-azacytidine induced reactivation of HPRT
| Clone * | TIMP1 | ARAF1 | ELK1 | ZNF41 | ZNF157 |
| t11-az-4 | - | - | - | - | + |
| t11-az-5 | - | - | - | - | + |
| t11-az-6 | + | - | + | - | - |
| t11-az-7 | + | - | + | - | - |
| t11-az-8 | + | - | + | - | - |
| t11-az-9 | + | - | + | - | + |
| t11-az-10 | + | + | - | + | + |
| t11-az-11 | - | - | - | + | + |
| t11-az-14 | - | - | - | - | + |
| t11-az-16 | - | - | - | - | + |
| t11-az-17 | - | - | - | - | - |
| t11-az-18 | - | - | - | + | + |
| t11-az-19 | + | - | + | - | - |
| t11-az-20 | - | - | - | - | - |
| t11-az-21 | - | - | - | - | - |
*The clones in bold were ones that were further subcloned to assess stability (see Table 2).
Despite 5-azacytidine treatment and subsequent expression in some clones, DNA methylation continued to be observed at 5' end of ARAF1 and TIMP1 in these clones, as shown in Figure 2C. Given our previous results demonstrating instability of expression in the presence of methylation, four clones were subcloned after 7 weeks of culture. Analysis of expression and DNA methylation for TIMP1 (Table 2) showed that, as expected, all subclones of a non-expressing clone (t11-az-4) remained silent. For both t11-az-8 and 9, the majority of clones lacked TIMP1 expression. Only two weakly positive clones were identified for each line, and even these subclones lost expression by 12 weeks in culture. Three subclasses of hybrids were observed for the t11-az-10 subclones, reminiscent of the situation seen in hybrids from females who spontaneously express TIMP1 from the inactive X. t11-az-10 was the only reactivated clone that expressed ARAF1, and nine of the 14 TIMP1-expressing subclones expressed ARAF1. The single subclone that was unmethylated at TIMP1 (t11-az-10-10) expressed TIMP1, and also expressed ARAF1, which was also unmethylated. t11-az-10-7 was methylated yet expressing for both TIMP1 and ARAF1. These clones (t11-az-10-10 and t11-az10-7) were further subcloned. The TIMP1 expression level of the subclones was determined by RNase protection. The t11-az-10-7 subclones retained DNA methylation, and expressed a lower level of TIMP1 than the unmethylated t11-az-10-10 subclones (Figure 2D).
Table 2.
Stability of methylation and expression of TIMP1 in subclones of four 5-azacytidine induced HPRT reactivants
| Subclones | t11-az-4 (-/M) | t11-az-8 (+/M)a | t11-az-9 (+/M)a | t11-az-10 (+/M) |
| TIMP-, methylated (-/M) | 10 | 10 | 15 | 3 |
| TIMP+, Methylated (+/M) | 0 | 2a | 2a | 13 |
| TIMP+, Unmethylated (+/U) | 0 | 0 | 0 | 1 |
aExpression in these branches was initially weak and became silent after time in culture. ELK1 expression was initially retained in one of the t11-az-9 TIMP1+ clones but was not analyzed after TIMP1 expression was lost. The expression of ZNF41/157 was not examined in these subclones.
DNA methylation analysis was routinely based on methylation-sensitive restriction enzyme digestion followed by PCR that examined 4 sites, 3 of which were over 50 bp downstream of the transcription start site (Figure 2E). To examine methylation of additional CpG dinucleotides closer to the promoter, methylation analysis by bisulfite modification followed by sequencing of PCR products was performed. Three additional CpGs near the TIMP1 transcription start yielded the same methylation pattern as had been observed at the restriction enzyme sites. Direct sequencing of the PCR product was performed to allow the identification of heterogeneous populations. This approach is complementary to the methylation-sensitive restriction enzyme approach, which gave a positive signal (amplification) for low levels of methylated DNA. The high background corresponding to the unconverted (methylated) Cs, presumably due to their under-representation in the sequence, prohibits identification of low levels of methylated bases by direct sequencing. However, no background of the converted base (A) was present and thus this technique is sensitive to the presence of low levels of unmethylated DNA. If the peak corresponding to the converted base (A) was demonstrably higher than the unconverted (G), the site was called unmethylated (white circles in Figure 2E). Completely methylated sites (as seen for the inactive X hybrid) are indicated by filled in black circles. Sequencing also yielded a mix of unmethylated (converted) and methylated (unconverted) sites. This was observed in a female cell line, where the methylated (unconverted – G) peak height was greater than or equal to the unmethylated (converted – A) peak height. Such a mix, demarcated by the grey circles, was also observed for the t11-az-10-7 cells, although in this case the unmethylated peak was consistently lower than the methylated peak.
DNase sensitivity of TIMP1 in somatic cell hybrids
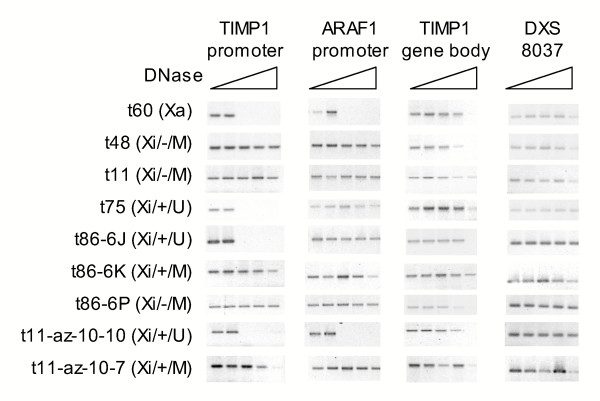
Nuclease sensitivity of the TIMP1 promoter, the promoter of the nearby ARAF1 gene, as well as the TIMP1 gene body and the anonymous DNA marker DXS8037 was assessed in a series of these clones (Figure 3). The promoters of TIMP1 and ARAF1 were sensitive to nuclease on the active, but not the inactive X chromosome, while the gene body or intergenic regions were generally resistant on both active and inactive X chromosomes. The TIMP1 promoter was also sensitive to digestion in the unmethylated expressing (Xi/+/U) TIMP1 clones, while it was insensitive in both the TIMP1-expressing, and silent methylated clones (Xi/+/M and Xi/-/M). A similar effect was observed for the demethylated clones, with both TIMP1 and ARAF1, being expressed in both the t11-az-10-10 and t11-az-10-7 clones, but only sensitive to nuclease digestion in the t11-az-10-10 clone, where they were unmethylated.
Figure 3.
Nuclease sensitivity of TIMP1. Nuclei from various cell lines (as indicated on the left) were treated with increasing amounts of DNase I (0, 0.1, 0.25, 0.5, 1.0 units) as represented by the increasing breadth of the triangle. The DXS8037 primers flank a non-coding region and were used to check that equal digestion and PCR amplification occurred across all cell lines. The cell line designations indicate X activity, TIMP1 expression status, and methylation status as shown in Figure 2B.
Histone modifications
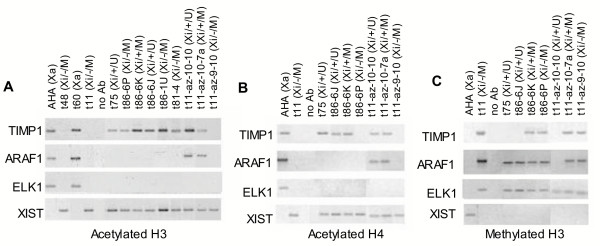
A growing number of specific modifications to the histone tails have been associated with both constitutive and facultative heterochromatin. To assess the potential role of such modifications in the escape from inactivation of TIMP1, we analysed the hybrids by ChIP with antibodies to acetylated histone H3 (antibody recognizes acetylated K9 and 14), acetylated histone H4 (antibody recognizes acetylated K 5, 8, 12, and 16) and methylated histone H3 (antibody to di-methyl K9, although the antibody will cross-react with tri-methyl K27 see: http://www.upstate.com/browse/productdetail.asp?ProductId=07-212). The promoter regions of TIMP1, ARAF1, ELK1 and the XIST gene (the latter is expressed solely from the inactive X chromosome) were analysed (shown in Figure 4). Additional analyses of the promoters of the PGK1 gene that is expressed only from the active X chromosome, and the ZFX gene that is expressed from both active and inactive X chromosomes, gave the anticipated results for active and inactive X chromosomes (data not shown). Antibody to acetylated H3 immunoprecipitated the TIMP1, ARAF1 and ELK1 promoters for the active, but not inactive X-containing hybrids, while the reverse was observed for the XIST promoter. ELK1 was not expressed in the inactive X hybrids examined, and was not immunoprecipitated. However for TIMP1 and ARAF1 association with acetylated histone H3 was observed for all expressing clones whether on the active or inactive X chromosome. In addition, immunoprecipitation of the TIMP1 promoter was observed for Xi/-/M clones (t86-6P/t86-1U and t81-4). Immunoprecipitation was completely concordant with expression for the acetylated histone H4 antibody at all of the genes examined. Methylation of H3 at K9 corresponds to silent chromatin, and thus, as anticipated, results were generally opposite to those seen for acetylation. Immunoprecipitation was observed when the gene was silent – i.e. XIST on the active X and TIMP1, ARAF1 and ELK1 on the inactive X. However, in all cases where there was expression in the presence of ongoing promoter methylation (for both ARAF1 and TIMP1) the promoter was immunoprecipitated by the methylated H3 antibody. Thus we detect a distinctive pattern of histone acetylation and methylation that does not correspond simply with the expression or DNA methylation status of the TIMP1 gene.
Figure 4.
ChIP analysis of TIMP1 expressing or non-expressing hybrid clones. PCR products were amplified from DNA at various X-linked gene promoters after ChIP using antibodies to the modifications listed below the panels for the cell lines listed across the top of each panel (see Figure 2B for derivation of lines). t11-az-10-7a is a subclone of t11-az-10-7 (see Figure 2D). The DNA template for 'no Ab' lanes was prepared following the ChIP procedure without an antibody. A. PCR amplification products after ChIP with acetylated histone H3. Similar analysis to A, using antibody to acetylated histone H4 (panel B), or methylated histone H3 (C).
Discussion
The facultative heterochromatin of the inactive X chromosome is a fascinating system to study the epigenetic modifications associated with silent chromatin as both an active and inactive version of most X-linked genes exist in female cells. However, not all genes on the 'inactive' X chromosome are subject to silencing [26], and these genes that escape inactivation must somehow maintain an active state on an otherwise silent chromosome. TIMP1 is subject to inactivation in many females, and we now show that when subject to inactivation the anticipated features of inactive chromatin are assembled – promoter DNA hypermethylation, nuclease insensitivity and histone methylation and hypoacetylation. In other females, however, we have previously demonstrated that TIMP1 continues to be expressed from the inactive X chromosome, and in this study we have exploited such chromosomes to analyze the epigenetic features that are associated with expression from the inactive X. By comparing expression of TIMP1 that occurs naturally with that induced by the demethylating agent 5-azacytidine we are also able to propose which feature may predispose to expression of TIMP1 in otherwise silent chromatin. We observed that expression of TIMP1 was associated with an active chromatin structure, despite the presence of the gene on the inactive X chromosome, except in three situations.
First, in female cell lines with or without TIMP1 expression from the inactive X there was a very similar extent of replication asynchrony, suggesting that the expression of TIMP1 was ocurring from a late-replicating region of the X chromosome. This is not surprising, as the genes flanking TIMP1, which are not variable in their inactivation, are located within 55 kb, so it is likely that at least one of ARAF1 or ELK1 shares a replication origin with TIMP1. We assessed replication timing by FISH, and detected a frequency of replication asynchrony for the HPRT locus in close agreement with the frequencies previously reported for this gene [27]. It has been suggested that the 'double dot' versus 'single dot' pattern may reflect chromatid association in addition or instead of replication asynchrony [17]. Regardless of the underlying cause, the results were highly reproducible and consistently showed less asynchrony for the TIMP1 locus relative to the HPRT locus, although still in the range that is generally considered asynchronous [28]. This reduced asynchrony at TIMP1 could reflect, or contribute to, weaker epigenetic silencing being established in this region of the X chromosome. There was a slight, but not statistically significant, trend for the female showing expression of TIMP1 from the inactive X chromosome to show even less replication asynchrony for TIMP1, however it seems unlikely that such a small difference could contribute substantially to TIMP1 expression from the inactive X, and thus other factors must be involved in permitting the expression of TIMP1 from the inactive X.
The other two situations in which expression and active chromatin features were not concordant were found in the somatic cell hybrids, and these results are summarized in Figure 5. As shown in the grey box, DNA methylation and additional features of silent chromatin were detected along with expression in the Xi/+/M (t86, t81) cells. We attribute this to heterogeneity in the population of cells, as subcloning yielded both methylated/silent and unmethylated/expressing clones. In addition to DNA methylation, these clones were observed to be insensitive to DNase and have methylated H3K9 residues near the TIMP1 promoter. As the assays for these features relied on PCR, a positive signal could be obtained from a subpopulation of cells with a silent chromatin structure, while another population of cells could be positive for expression and active chromatin modifications (histone acetylation). We also suggest that heterogeneity accounts for expression in the presence of DNA methylation in several of the demethylated clones, including the t11-az-10-7 clone. However, unlike the mixed population of subclones obtained with all other Xi/+/M cells, eight of eight subclones of t11-az-10-7 were methylated and expressing, and six of these subclones examined by RPA showed a consistently reduced level of expression relative to their unmethylated counterparts. While it was surprising that subcloning did not isolate distinct subpopulations, the presence of an unmethylated subpopulation was detectable by bisulphite sequencing. Thus heterogeneity again seems the most likely explanation for DNA methylation, DNase insensitivity and histone H3K9 methylation in these expressing cells. However, without single cell assays it is not possible to rule out that there are methylated cells that express TIMP1 at reduced levels and show additional features of silent chromatin. These two classes of clones were the only exceptions to concordance between nuclease sensitivity and gene expression at the promoters of the TIMP1 and ARAF1 genes; and previous studies of the nucleosomal organization of the HPRT1 gene promoter have shown that methylation does not directly affect the differential positioning of nucleosomes on active and inactive X-linked promoters [19]. Thus we believe that the methylated/expressing/nuclease insensitive clones reflect the presence of a subpopulation of silent cells. This heterogeneity demonstrates the unstable nature of silencing for TIMP1 in these cells.
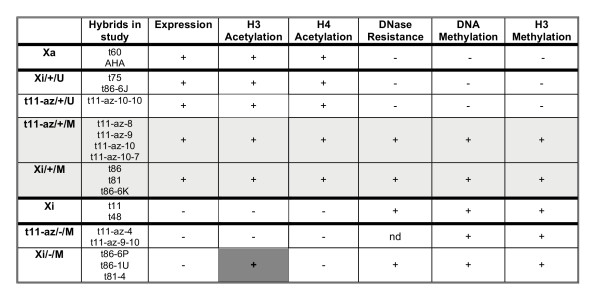
Figure 5.
Summary of chromatin features observed in somatic cell hybrid clones for the TIMP1 gene. Most subclones and demethylated clones follow the patterns seen for the active and inactive X hybrids that are outlined in bold. A positive (+) designates the presence of the feature, while a negative (-) depicts the absence of the feature, while ND means that the feature has not been examined in that class of clones. The assays used are PCR-based and would detect a small population of cells. DNase sensitivity is listed as the inverse – DNase resistance – as it is the presence of undigested (resistant) DNA that will yield a 'positive' PCR signal. In each category of clones not all clones have been examined for all features. For the clones shaded in grey the results are an amalgamation of results anticipated from an active and inactive X, and we suggest these clones represent a mixed population. This suggestion is generally supported by sub-cloning experiments (see text for discussion). The ongoing H3 acetylation of the Xi/-/M hybrids (highlited in darker grey) cannot be attributed to heterogeneous cell populations, and since it is not seen for demethylated hybrids that have lost TIMP1 expression (t11-az/-/M) we suggest that this modification reflects a predisposing feature of inactive X chromosomes that express TIMP1.
The third exception is the most interesting, and is highlighted in Figure 5 with a dark grey fill. Acetylation of histones is generally seen for active genes, and acetylated histone H4 showed complete concordance with expression for all genes. However, the Xi/-/M clones (t86-6P and 1 U as well as t81-4) showed ongoing acetylation at H3 despite having lost TIMP1 expression from the inactive X chromosome. The Xi/-/M subclones were derived from two different unstable, naturally expressing inactive X chromosomes (in t86 and t81), however expression of TIMP1 in these hybrids was now stably silenced, and thus this result was not a reflection of a mixed population of cells. Furthermore, acetylation cannot simply reflect a failure of this region to reset chromatin structure, as the demethylated t11-az-9-10 hybrid which had lost expression had also lost acetylation. Methylation of H3 K9 was observed in these silent clones, so the Xi/-/M cells, despite being a homogeneous population, appear to show both methylation and acetylation of histone H3. This may reflect the lack of specificity of the antibodies used for these experiments, as the acetylated H3 antibody recognizes acetylation at residues 9 and 14, while the antibody to methylated K9 can cross-react with methylation of K27. Thus immunoprecipitation by both antibodies may reflect modification of specific lysines. It is also possible that only a particular set of nucleosomes show the acetylation mark, and as the sonicated fragments immunoprecipitated in the assay averaged ~600 bp, modifications on several different nucleosomes flanking the primers used could result in immunoprecipitation. Regardless of the specific site of modification, acetylation of histone H3 was the one feature that was consistently associated with X chromosomes that were naturally predisposed to expression from the inactive X chromosome, regardless of expression status. Thus we propose that histone H3 acetylation differs at the TIMP1 genes in females, predisposing some females to expression from the inactive X. Unfortunately this hypothesis is difficult to test, as females generally show acetylation at TIMP1, due to the presence of the active X chromosome. It would be necessary to study clonal populations of cells from a female with a polymorphism close enough to the promoter to be analysed by ChIP, and currently no such polymorphisms are known. Our previous work did not show an association between expression of TIMP1 and a downstream polymorphism in exon 5 [12].
A different predisposing epigenetic mark for expression from the inactive X has previously been reported in mice. Methylation of H3 at K4 was restricted to the promoter in undifferentiated embryonic stem cells for genes that would subsequently be expressed monoallelically (i.e. genes subject to X inactivation or imprinting), while autosomal genes or genes that escape inactivation had H3 K4 methylation in the gene body as well as the promoter [11]. Such methylation was observed in the body of the Smcx gene which is initially silenced, and then reactivates, reminiscent of the H3 acetylation mark and instability of silencing that we observe for TIMP1. While many genes escape inactivation in humans, TIMP1 provides us with a unique opportunity to examine the gene in its expressed and silent state in hybrid somatic cells. Thus, it will be interesting to study additional chromatin modifications for TIMP1, including H3 K4 methylation, as well as additional modifications recently associated with the inactive X chromosome such as H4 K20 methylation [29] or H2A ubiquitinylation [30,31]. Even more interesting would be to determine if the changes seen are observed for additional X-linked genes that are variable in their inactivation status, if suitable model systems could be developed.
Previous examinations of genes that escape inactivation have shown an active chromatin structure (reviewed in [7]), and recent results further demonstrate that epigenetic modifications seem to be heterogeneous in their distribution along the X [32]. These 'flavours' of inactive X chromatin may correspond to clusters of genes that escape inactivation. While genes that escape inactivation tend to be clustered in blocks and enriched on the short arm of the X chromosome, genes such as TIMP1 that are variable in their inactivation are more evenly distributed along the X chromosome ([5], reviewed in [6]), and thus may reflect different mechanisms leading to expression from the inactive X. The CTCF boundary factor has recently been shown to be found between the domain of escape and that of inactivation [33]. It is not known whether such boundaries flank the variably inactivated genes. The five clones that showed reactivation of ELK1 were all TIMP1+, suggesting a co-ordinate reactivation between the two genes. Only one ARAF1 reactivant was identified, and this clone was also TIMP1+. However, this co-ordinate reactivation of TIMP1 with flanking gene(s) is different from the TIMP1-specific expression seen for the inactive X naturally. The ZNF157/41 genes did not seem to show co-ordinate reactivation with the TIMP1 region, however all three ZNF41 positive clones were ZNF157 positive, which suggests they may be in a separately controlled domain.
While association with a CpG island does not differ between genes subject to or escaping from inactivation, genes heterogenous in their inactivation are more likely to lack an island [5], consistent with the TIMP1 promoter being the least CpG dense of the region. Although TIMP1 lacks a CpG island, our analysis suggests that methylation of the few CpGs present near the promoter generally correlates well with silencing. Demethylation resulted in at least transient expression of all genes examined, confirming the importance of DNA methylation in stable maintenance of X chromosome silencing. Examination of the ELK1, TIMP1 and ARAF1 promoters after demethylation showed the presence of DNA methylation, even when the clones were expressing the genes. The majority of these clones subsequently resilenced these genes, as has been observed for other genes following demethylation [34], perhaps reflecting only partial initial demethylation or the spread of silencing from adjacent silent regions that retained DNA methylation or other epigenetic marks of silencing.
It has been proposed that the evolutionarily recent addition of the X short arm may predispose genes located there to escape from silencing [35]. Interestingly, TIMP1 is very close to the evolutionary breakpoint between the region added to the X after marsupial divergence. ARAF1 is found on the marsupial X while TIMP1 and its surrounding gene SYN1 are autosomal in marsupials, suggesting that they are part of the eutherian addition to the human X [36]. Since genes closely flanking TIMP1 are normally subject to X inactivation, even when TIMP1 is expressed, evolutionary history alone cannot explain the escape from inactivation, although it may result in a different genomic context that contributes to expression from the inactive X. However, inspection of genomic features in the region surrounding TIMP1 does not show a substantial difference in the frequency of repetitive elements near TIMP1 relative to the flanking ARAF1 and ELK1 genes (Figure 2A).
Conclusion
Several factors, including reduced replication asynchrony, lower CpG density, and more recent evolutionary addition to the X, may contribute to less stringently controlled inactivation for TIMP1. However, we propose that there is a difference between females for a feature unique to TIMP1 that predisposes some females to expression of the gene. This mark appears to be at least reflected in a difference in acetylation of histone H3 on the inactive X in females predisposed to expression of TIMP1 from the inactive X chromosome. While histone acetylation appears to be a predisposing mark for expression of TIMP1, there may be a different hierarchy of epigenetic modifications permitting expression of other genes from the inactive X. Elucidation of these mechanisms is important not only as a model for epigenetic gene regulation but because genes that escape inactivation contribute to the phenotype of X chromosome anueploidies, and may also result in differential male/female expression levels and disease susceptibilities.
Methods
Cell culture
Lymphoblast cell lines were grown in RPMI 1640 media (Stem Cell Technologies) supplemented with 15% fetal calf serum (Cansera), L-glutamine (Invitrogen) and penicillin/streptomycin (Invitrogen). Cells were harvested 12–26 hours after addition of fresh media by centrifugation. The human/rodent somatic cell hybrids were grown in alpha minimal essential media (Invitrogen) supplemented with 7.5% fetal calf serum, L-glutamine, penicillin/streptomycin, and non-essential amino acids (Invitrogen) to sub-confluence before harvesting with trypsin-EDTA (0.25%). Cell lines have been previously described [14]. To generate single cell clones, the hybrid cultures were plated to a final concentration of 3 to 17 cells/60 mm plate. After 5 to 10 days in culture, well-separated colonies were isolated by trypsinization in cloning cylinders and transferred to new 60 mm plates. To induce demethylation, an inactive X-containing hybrid that had never expressed TIMP1 (t11-4Aaz-5) was treated with 5-azacytidine as previously described [37]. The cells were grown in media supplemented with HAT (Invitrogen) to select for HPRT reactivants and then single cell cloned to test for TIMP1-positive cultures. To remove any confounding effects of HPRT selection, the cells were transferred back to alpha minimal essential media after 2 weeks of selection. The expression of genes in the TIMP1 region was determined by RT-PCR as described previously [12].
Replication timing
Approximately 4 × 106 lymphoblast cells were harvested one day after subculture to ensure that they were actively growing. The cell pellet was resuspended in 8 ml of prewarmed hypotonic (0.75 M KCl) and then incubated at 37C for 10 minutes. 2 to 3 ml of fixative (3:1 methanol:glacial acetic acid) was slowly added before spinning at 200 g for 10 minutes. The cell pellet was washed three more times with 5 ml fixative and then resuspended in 10 ml fixative for storage at -20C for up to a week. The nuclei preparations were dropped onto slides and left at room temperature overnight. The slides were then incubated in 2X SSC at 37C for 30 minutes followed by 2 minute room temperature incubations in each of: 70%, 85%, and 95% ethanol, and then allowed to air dry. To denature, the slides were incubated in fresh 70% formamide/2 × SSC at 74C for 2 minutes followed by an ice cold ethanol series of rinses (70%, 85%, 95% for 2 minutes each) and air drying. Probes for both the TIMP1 locus (lambda phage TIMP-3.9X) and the HPRT locus (Hulambda4x-8, ATCC 57236) were labelled with dUTP-digoxigenin (Roche 1745816) by nick translation (Roche 0976776). Unincorporated nucleotides were removed with a PCR clean-up kit (Qiagen), and 1 ul (approximately 100 ng) of labelled probe was mixed with 10 ul of 70% formamide hybridization buffer and 40 ng of human Cot-1 DNA (Invitrogen 15279011). The probes were denatured at 74C for 10 minutes, pre-annealed at 37C for one hour and then added to the prepared slide to hybridize overnight in a humidified chamber at 37C. The slides were washed in 50% formamide/2 × SSC for 15 minutes at 43C, followed by two washes in 2 × SSC for 4 minutes at 37C and then 3 washes at room temperature for 2 minutes each in 1 × PBD (0.1 M NaH2PO4, 0.1 M Na2HPO4, 0.1% Triton X). To visualize the probe, slides were incubated with 500 ng of anti-DIG (sheep – Roche 1207741) conjugated to fluorescein, for 5 minutes at 37C, followed by three two-minute washes in 1 × PBD. To amplify the signal, FITC-anti-sheep (IgG FI-6000 (Vector Laboratories)) was incubated and washed as above. The slides were then counterstained with DAPI mixed with antifade (VectorLabs). Cells were scored for nuclei with single-single, single-double, or double-double TIMP1 or HPRT signals on a Ziess Axioplan II microscope by a single individual who was blinded as to the cell line being analysed.
Methylation analyses
For methylation-sensitive restriction enzyme analysis, genomic DNA was pre-digested with EcoRI at 37C overnight, followed by incubation with 2 ul of RNase at 37C for 15 minutes. After phenol extraction and ethanol precipitation, the DNA was quantified by spectrophotometry. Pre-digested DNA (2 ug) was then incubated overnight at 37C in a total volume of 20 ul with 20 units of one of the following: mock enzyme (uncut), HpaII, or HhaI. An aliquot of 1 ul (100 ng) was then used as a template in the PCR reactions as described previously [14]. All primers flanked a region of genomic DNA that did not contain EcoRI restriction enzyme sites and contained 1–2 HpaII or HhaI sites. For bisulfite analysis, 500 ng of genomic DNA was first denatured with 3 ul of 3 M NaOH at 37C for 10 minutes. After 15 ul of 20 mM hydroquinone and 255 ul of 3.9 M sodium bisulfite were added and mixed well, reactions were left at 50C for 16 hours to allow the unmethylated cytosines to convert to uracil. DNA was purified using the DNA Wizard Clean-Up Kit (Promega). After amplification with the TIMP-S primers (see Table 3), the 3' reverse oligonucleotide was used as the primer for sequencing of the PCR product.
Table 3.
Primers for PCR analyses
| Primer pair | Sequence | Use |
| TIMP1 5' (promoter) [14] | 5'A: CCCTTGGGTTCTGCACTGA* 5'B: CCAAGCTGAGTAGACAGGC |
Methylation ChIP DNase sensitivity |
| TIMP1 CA (gene body) [12] | CA1: GGGTTCCAAGCCTTAGGGGA CA2: AGGCTGTTCCAGGGAGCCGC |
DNase sensitivity |
| TIMP1 S (bisulfite) | 5S: GttttTTGGtTTtTGtAtTGATGGT 3S: CCAAaCTaAaTAaACAaaCATCTAaC** |
Bisulfite sequencing |
| ARAF1 M1:M4 (promoter) [14] | M1: TGCCAAAGCCCTAAGGTCA M4: CGCTGTCGACGATGGTCT M3: GTGAGGAAACAAGAAGAGAG |
Methylation ChIP DNase sensitivity |
| XIST 3':5' (gene body) [39] | 3':GAAGTCTCAAGGCTTGAGTTAGAAG 5': TTGGGTCCTCTATCCATCTAGGTAG |
Methylation DNase sensitivity |
| XIST A5:29r (promoter) [37] | A5: TTTCTTACTCTCTCGGGGCT 29r: ATCAGCAGGTATCCGATACC |
ChIP |
| ELK1 5' (promoter) [14] | A: GCACAGCTCTGTAGGGAA B: AGCTCACCTGTGTGTAGCG |
Methylation ChIP |
| STA A:B (intergenic) | A: CACCTGTGTGTCATGTATAC B: CCAGTATTGGTCTTCCAGTT |
DNase sensitivity |
| 8037 A:B (intergenic) | A: GAGGCAAGACATCCATTCC B: TGACTTTGAGCGAGCAGGT |
Reference region |
* There is a mismatch in the TIMP 5'A primer, the underlined G should be C.
** The lower-case letters in the primers are the bases modified by the bisulfite reaction. All C nucleotides should have been converted because there were no CpG pairs with possible protective methylation.
RNase Protection quantitation of TIMP1 levels
RNase protection analysis was performed with Ambion's RPA II kit, following the manufacturer's directions. RNA probes were isolated after in vitro transcription with 32P-UTP. After solution hybridization overnight of excess antisense radiolabelled probe to 10 ug of total RNA, any unhybridized probe and sample RNA was removed by RNase digestion. The hybridized product was then separated on a native 5% polyacrylamide gel, visualized by autoradiography, and bands quantified by phosphoimager (BioRad FX). The intensity of the TIMP1 fragment was compared to the intensity of the band detected for MIC2 used to control for the amount of input RNA. All RPA results were normalized to one stock RNA to decrease variability between gels (see [14]).
DNaseI sensitivity
The protocol for nuclease sensitivity was adapted primarily from [38]. The somatic cell hybrids were grown to 75% confluence on 60 mm plates before harvesting with 0.25% trypsin-EDTA. The cell pellets were resuspended in 300 ul ice-cold DNase buffer (0.3 M sucrose, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, and 15 mM Tris-HCL pH7.5). The suspensions were then split into 6 tubes of 50 ul each and another 50 ul of ice-cold DNase buffer with 0.4% Nonidet P40 was added. The tubes were mixed gently and placed on ice for four to five minutes before 100 ul of freshly diluted DNaseI (Invitrogen 18047-019) was added, such that 2.5 U, 1 U, 0.5 U, 0.25 U, 0.1 U, and no enzyme were used. The reaction was then incubated at 25C for 5 minutes, followed by 95C for 15 minutes. The DNA was isolated with a standard phenol/chloroform extraction protocol, quantified with spectrophotometry, and diluted to 60 ng/ul, 12 ng/ul, and 4 ng/ul. 1 ul was used as a PCR template with primers listed in Table 3. The diluted DNAs showed product intensity inversely proportional to concentration, indicating that the PCRs at 25 cycles of amplification were in the linear range of amplification, and the results shown in figure 3 are for the 12 ng/ul template.
Chromatin Immunopreciptation Assay (ChIP Assay)
The hybrid cells were grown in a t25 flask and were harvested after treatment with 5 drops of 0.25% trypsin-EDTA for 2 minutes at room temperature, and then washed in 1 × PBS. The cells were placed in a 1.5 ml tube with one ml of 0.37% formaldehyde in minimal essential media (Invitrogen) and incubated at 37C for ten minutes. From this point, the cells were kept on ice. The cells were washed twice with 1/100 proteinase inhibitor cocktail (Sigma) in 1 × PBS and then centrifuged at 2500 rpm for 4 minutes at 4C. The pellet was resuspended in 200 ul SDS lysis buffer (Upstate) with 1/100 proteinase inhibitor cocktail and placed on ice for 10 minutes. The suspension was drawn up with a 25 gauge needle and then sonicated. Immunoprecipitation was performed (Upstate Biochemicals catalogue number 17-295) with the following antibodies: Anti-acetyl H3 against lysine 9 and 14 (catalogue number 06-599); Anti-acetyl H4 against lysine 5, 8, 12, and 16 (catalogue number 06-866); and Anti-dimethyl-H3 against lysine 9 (catalogue number 07-212).
Abbreviations
H3 – histone H3; H4 – histone H4; K – lysine; ChIP – chromatin immunoprecipitation; X – X chromosome; Y – Y chromosome; Xi – inactive X; Xa – active X; M – methylated; U – unmethylated; + expressing; - not expressing; S – single signal; D – double signal.
Authors' contributions
CA carried out the molecular genetic studies and data analysis and drafted the manuscript. CB conceived of the study, participated in the experimental design and data analysis and wrote the final manuscript. Both authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This study was supported by a Canadian Institutes of Health Research operating grant (MOP13690).
Contributor Information
Catherine L Anderson, Email: catherine.anderson@shaw.ca.
Carolyn J Brown, Email: cbrown@interchange.ubc.ca.
References
- Cheung VG, Conlin LK, Weber TM, Arcaro M, Jen KY, Morley M, Spielman RS. Natural variation in human gene expression assessed in lymphoblastoid cells. Nat Genet. 2003;33:422–425. doi: 10.1038/ng1094. [DOI] [PubMed] [Google Scholar]
- Whitney AR, Diehn M, Popper SJ, Alizadeh AA, Boldrick JC, Relman DA, Brown PO. Individuality and variation in gene expression patterns in human blood. Proc Natl Acad Sci U S A. 2003;100:1896–1901. doi: 10.1073/pnas.252784499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson MW, McInnes RR, Willard HF. Genetics in Medicine. 5. Philadelphia, W.B. Saunders Co.; 1991. [Google Scholar]
- Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Greally JM. A stain upon the silence: genes escaping X inactivation. Trends Genet. 2003;19:432–438. doi: 10.1016/S0168-9525(03)00177-X. [DOI] [PubMed] [Google Scholar]
- Disteche CM, Filippova GN, Tsuchiya K. Escape from X inactivation. Cytogenet Genome Res. 2002;99:36–43. doi: 10.1159/000071572. [DOI] [PubMed] [Google Scholar]
- Craig IW, Harper E, Loat CS. The genetic basis for sex differences in human behaviour: role of the sex chromosomes. Ann Hum Genet. 2004;68:269–284. doi: 10.1046/j.1529-8817.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KD, Greally JM, Yi Y, Noel KP, Truong JP, Disteche CM. Comparative sequence and X-inactivation analyses of a domain of escape in human Xp11.2 and the conserved segment in mouse. Genome Res. 2004;14:1275–1284. doi: 10.1101/gr.2575904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingenfelter PA, Adler DA, Poslinski D, Thomas S, Elliot RW, Chapman VM, Disteche CM. Escape from X inactivation of Smcx is preceded by silencing during mouse development. Nat Genet. 1998;18:212–213. doi: 10.1038/ng0398-212. [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Navarro P, Avner P. Promoter-restricted H3 Lys 4 di-methylation is an epigenetic mark for monoallelic expression. Hum Mol Genet. 2003;12:3343–3348. doi: 10.1093/hmg/ddg351. [DOI] [PubMed] [Google Scholar]
- Anderson C, Brown CJ. Polymorphic X-chromosome inactivation of the human TIMP1 gene. Am J Hum Genet. 1999;65:699–708. doi: 10.1086/302556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L, Willard HF. Heterogeneous gene expression from the inactive X chromosome: an X-linked gene that escapes X inactivation in some human cell lines but is inactivated in others. Proc Natl Acad Sci USA. 1999;96:7364–7369. doi: 10.1073/pnas.96.13.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CL, Brown CJ. Variability of X chromosome inactivation: effect on levels of TIMP1 RNA and role of DNA methylation. Hum Genet. 2002;110:271–278. doi: 10.1007/s00439-002-0676-8. [DOI] [PubMed] [Google Scholar]
- Plath K, Mlynarczyk-Evans S, Nusinow DA, Panning B. Xist RNA and the mechanism of X chromosome inactivation. Annu Rev Genet. 2002;36:233–278. doi: 10.1146/annurev.genet.36.042902.092433. [DOI] [PubMed] [Google Scholar]
- Willard HF, Latt SA. Analysis of deoxyribonucleic acid replication in human X chromosomes by fluorescence microscopy. Am J Hum Genet. 1976;28:213–227. [PMC free article] [PubMed] [Google Scholar]
- Azuara V, Brown KE, Williams RR, Webb N, Dillon N, Festenstein R, Buckle V, Merkenschlager M, Fisher AG. Heritable gene silencing in lymphocytes delays chromatid resolution without affecting the timing of DNA replication. Nat Cell Biol. 2003;5:668–674. doi: 10.1038/ncb1006. [DOI] [PubMed] [Google Scholar]
- Chow JC, Brown CJ. Forming facultative heterochromatin: silencing of an X chromosome in mammalian females. Cell Mol Life Sci. 2003;60:2586–2603. doi: 10.1007/s00018-003-3121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yang TP. Nucleosomes are translationally positioned on the active allele and rotationally positioned on the inactive allele of the HPRT promoter. Mol Cell Biol. 2001;21:7682–7695. doi: 10.1128/MCB.21.22.7682-7695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P, Turner B. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell. 1993;74:281–289. doi: 10.1016/0092-8674(93)90419-Q. [DOI] [PubMed] [Google Scholar]
- Heard E, Rougelle C, Arnaud D, Avner P, Allis CD, Spector DL. Methylation of histone H3 at Lys-9 is an early mark on the X chromosome during X inactivation. Cell. 2001;107:727–738. doi: 10.1016/S0092-8674(01)00598-0. [DOI] [PubMed] [Google Scholar]
- Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, de la Cruz CC, Otte AP, Panning B, Zhang Y. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- Gilbert SL, Sharp PA. Promoter-specific hypoacetylation of X-inactivated genes. Proc Natl Acad Sci U S A. 1999;96:13825–13830. doi: 10.1073/pnas.96.24.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeulle C, Chaumeil J, Sarma K, Allis CD, Reinberg D, Avner P, Heard E. Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol Cell Biol. 2004;24:5475–5484. doi: 10.1128/MCB.24.12.5475-5484.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hors-Cayla MC, Heuertz S, Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somat Cell Genet. 1983;9:645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci U S A. 1999;96:14440–14444. doi: 10.1073/pnas.96.25.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs BA, Chinault AC. Analysis of replication timing properties of human X-chromosomal loci by fluorescence in situ hybridization. Proc Natl Acad Sci. 1994;91:6083–6087. doi: 10.1073/pnas.91.13.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribnau J, Luikenhuis S, Hochedlinger K, Monkhorst K, Jaenisch R. X chromosome choice occurs independently of asynchronous replication timing. J Cell Biol. 2005;168:365–373. doi: 10.1083/jcb.200405117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A. A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol. 2004;2:E171. doi: 10.1371/journal.pbio.0020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, Nesterova TB, Silva J, Otte AP, Vidal M, Koseki H, Brockdorff N. Polycomb Group Proteins Ring1A/B Link Ubiquitylation of Histone H2A to Heritable Gene Silencing and X Inactivation. Dev Cell. 2004;7:663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Fang J, Chen T, Chadwick B, Li E, Zhang Y. Ring1b-mediated H2A ubiquitination associates with inactive X chromosomes and is involved in Initiation of X-inactivation. J Biol Chem. 2004;279:52812–5. doi: 10.1074/jbc.C400493200. [DOI] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF. Multiple spatially distinct types of facultative heterochromatin on the human inactive X chromosome. Proc Natl Acad Sci U S A. 2004;101:17450–17455. doi: 10.1073/pnas.0408021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippova GN, Cheng MK, Moore JM, Truong JP, Hu YJ, Nguyen DK, Tsuchiya KD, Disteche CM. Boundaries between chromosomal domains of X inactivation and escape bind CTCF and lack CpG methylation during early development. Dev Cell. 2005;8:31–42. doi: 10.1016/j.devcel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Gartler SM. 5-Azacytidine-induced reactivation of the human X chromosome-linked PGK1 gene is associated with a large region of cytosine demethylation in the 5' CpG island. Proc Natl Acad Sci U S A. 1990;87:4174–4178. doi: 10.1073/pnas.87.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahn BT, Page DC. Four evolutionary strata on the human X chromosome. Science. 1999;286:964–967. doi: 10.1126/science.286.5441.964. [DOI] [PubMed] [Google Scholar]
- Wilcox SA, Watson JM, Spencer JA, Graves JA. Comparative mapping identifies the fusion point of an ancient mammalian X-autosomal rearrangement. Genomics. 1996;35:66–70. doi: 10.1006/geno.1996.0323. [DOI] [PubMed] [Google Scholar]
- Tinker AV, Brown CJ. Induction of XIST expression from the human active X chromosome in mouse/human somatic cell hybrids by DNA demethylation. Nucl Acids Res. 1998;26:2935–2940. doi: 10.1093/nar/26.12.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Feil R. Analysis of chromatin in limited numbers of cells: a PCR-SSCP based assay of allele-specific nuclease sensitivity. Nucleic Acids Res. 1999;27:e32. doi: 10.1093/nar/27.22.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]