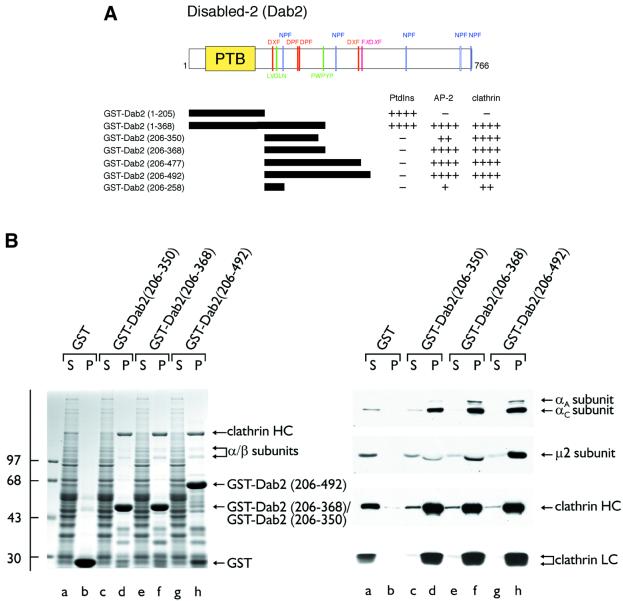
Fig. 1. Dab2 binds to both the AP-2 adaptor complex and clathrin. (A) Schematic illustration of the overall domain organization of Dab2 and the various GST–Dab2 constructs used. Phosphoinositide-, AP-2- and clathrin-binding properties of each fusion protein are indicated qualitatively. (B) Approximately 50 µg of either GST (lanes a and b), GST–Dab2(206–350) (lanes c and d), GST–Dab2(206–368) (lanes e and f) or GST–Dab2(206–492) (lanes g and h) immobilized on GSH–Sepharose were incubated with rat brain cytosol. After centrifugation, aliquots corresponding to 1/60 of each supernatant (S) and 1/5 of each washed pellet (P) were resolved by SDS–PAGE and either stained with Coomassie Blue or transferred to nitrocellulose. Portions of the blots were probed with the anti-AP-2 α-subunit mAb 100/2, anti-AP-2 µ2-subunit antiserum, the anti-clathrin heavy chain (HC) mAb TD.1 or the anti-clathrin light chain (LC) mAb Cl 57.3. The position of the molecular mass standards (in kDa) is indicated on the left and only the relevant portion of each blot is shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.