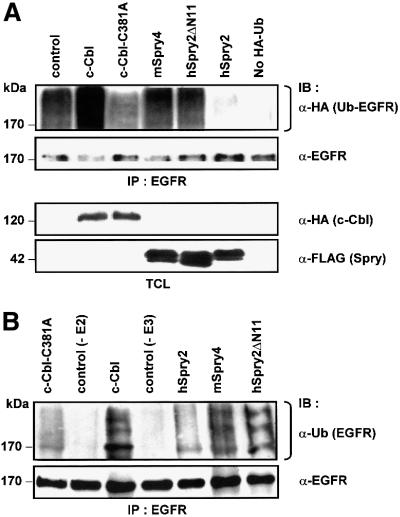
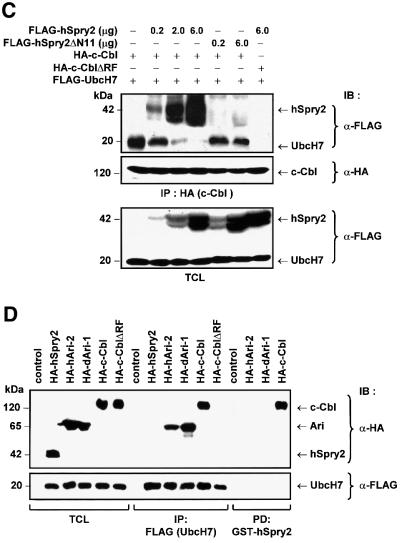
Fig. 3. hSpry2 abrogates c-Cbl-dependent ubiquitylation of EGFRs. (A) COS-1 cells were co-transfected with 1.0 µg HA-tagged ubiquitin and 3.0 µg each of either vector control, c-Cbl, c-Cbl-C381A or the different FLAG-tagged Spry forms per 100 mm culture dish. Forty-eight hours later, cell monolayers were treated with 100 ng/ml EGF at 37°C for 10 min. Total cell lysates (TCL) were subjected to immunoprecipitation (IP) using anti-EGFR–agarose-conjugated beads, immunoblotted (IB) with anti-HA to distinguish ubiquitin-conjugated EGFR, and anti-EGFR to assess the amounts of immunoprecipitated EGFR. TCL samples were analysed with anti-HA or anti-FLAG to show relative expression levels of the various constructs. The bracket indicates the position of high molecular weight species of ubiquitin-positive EGFRs. (B) Immunoprecipitated EGFR proteins were subjected to an in vitro ubiquitylation assay in the presence of purified UbcH7 (or no added UbcH7 as control without E2), eluted c-Cbl protein alone (or no added c-Cbl as control without E3), recombinant c-Cbl with either GST–hSpry2, GST–mSpry4 or GST–hSpry2ΔN11 fusion proteins, or c-Cbl-C381A fusion protein alone, plus the essential components in the ubiquitylation system. The reaction products were analysed following western blotting protocol with anti-ubiquitin. The bracket highlights the position of high molecular species of ubiquitin-positive EGFRs. (C) Competitive binding between hSpry2 and UbcH7 for the RING finger domain of c-Cbl. COS-1 cells (100 mm dishes) were co-transfected with 3.0 µg each of FLAG-tagged UbcH7, HA–c-Cbl or HA–c-Cbl-ΔRF mutant, and varying amounts of FLAG–hSpry2 or the c-Cbl non-binding truncation mutant FLAG–hSpry2ΔN11, as indicated. Serum-deprived cells were stimulated with 100 ng/ml EGF at 37°C for 10 min. TCL were subjected to precipitation using anti-HA and immunoblotted (IB) with anti-FLAG to detect associated hSpry2 or UbcH7, and anti-HA to show the relative amounts of immunoprecipitated c-Cbl. A TCL blot was probed with anti-FLAG to demonstrate quantitatively the expression levels of exogenous hSpry2 and UbcH7 proteins. (D) COS-1 cells (100 mm dishes) were untransfected (control), or co-transfected with 3.0 µg each of FLAG-tagged UbcH7 and either HA–hSpry2, HA–human Ariadne-2 (hARI-2), HA–Drosophila Ariadne-1 (dAri-1), HA–c-Cbl or HA–c-CblΔRF constructs. Immunocomplexes of FLAG–UbcH7 or ‘pull-downs’ using GST–hSpry2 were detected using anti-HA. Total cell lysates were analysed for equivalent levels of protein expression and normalized sample loading using antibodies as indicated. An immunoblot (with TCL loaded alongside) was probed with anti-HA to detect hSpry2-binding proteins, and with anti-FLAG to verify equal amounts of immunoprecipitated UbcH7.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.