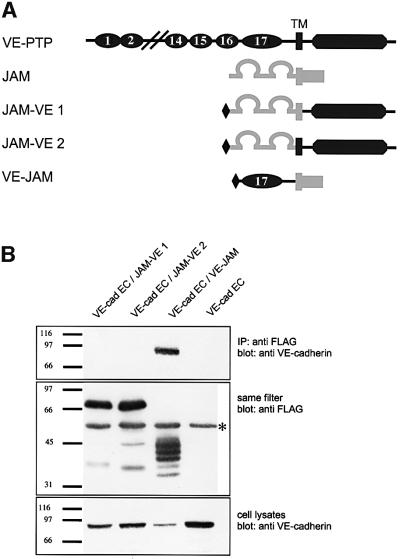
Fig. 4. The 17th FNIII repeat of VE-PTP is sufficient for binding to VE-cadherin. (A) Schematic representation of the domain structure of full-length VE-PTP (dark grey), full-length JAM (light grey) and three chimeric fusion proteins containing an N-terminal Flag-tag and various parts of VE-PTP and JAM: the cytoplasmic tail of VE-PTP and the extracellular and transmembrane domain of JAM (JAM–VE1), the cytoplasmic and transmembrane domain of VE-PTP fused to the extracellular domain of JAM (JAM–VE2) or the extracellular 17th FNIII repeat of VE-PTP and the cytoplasmic and transmembrane domain of JAM (VE–JAM). (B) COS cells were transfected with truncated VE-cadherin lacking its cytoplasmic tail (VE-cad EC, lanes 1–4) and, in addition, co-transfected with one of the three different VE-PTP– JAM fusion proteins (lanes 1–3). Proteins immunoprecipitated with anti-Flag antibodies were immunoblotted first with anti-VE-cadherin antibodies (top), and then the same filters were re-probed with anti-Flag antibodies (middle). Total cell lysates were immunoblotted with anti-VE-cadherin antibodies (bottom). Note that truncated VE-cadherin was only co-precipitated with the VE-PTP–JAM fusion protein that included the 17th FNIII repeat of VE-PTP (lane 3). The asterisk marks the IgG heavy chain detected by the secondary antibody. Molecular mass markers (in kDa) are shown on the left.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.