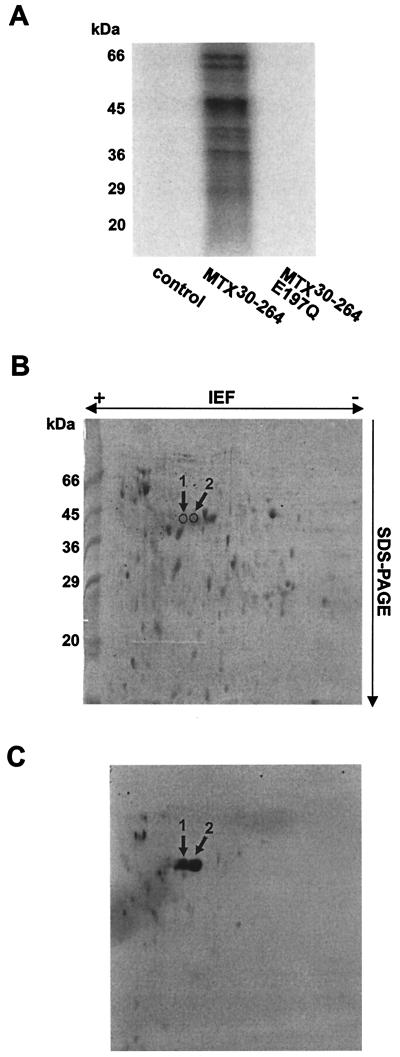
FIG. 1.
[32P]ADP-ribosylation of E. coli proteins by MTX30-264. (A) Total E. coli cell lysates (12 μg) were incubated with thrombin cleavage buffer (control), MTX30-264 (50 nM), or MTX30-264E197Q (50 nM) in the presence of 100 μM [32P]NAD. Proteins were separated by SDS-PAGE, and labeled proteins were detected by phosphorimaging (shown). (B and C) E. coli cell lysates (150 μg) were incubated with MTX30-264 (1 μM) and [32P]NAD (100 μM) and subjected to two-dimensional gel electrophoresis. Proteins were detected with Coomassie blue (B) and then subjected to autoradiography (C). Two ∼45-kDa proteins (arrows) were excised from the gel and analyzed by MS, resulting in the identification of EF-Tu (Table 1) as a protein target for MTX.