Abstract
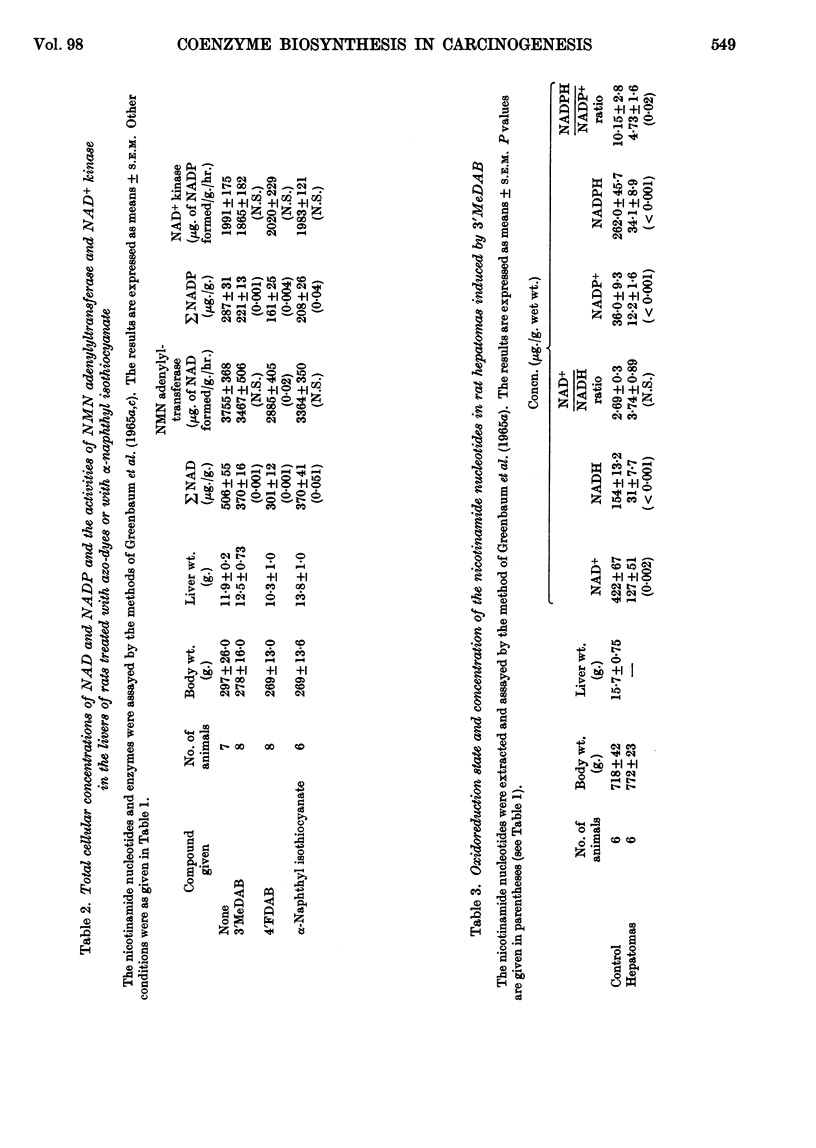
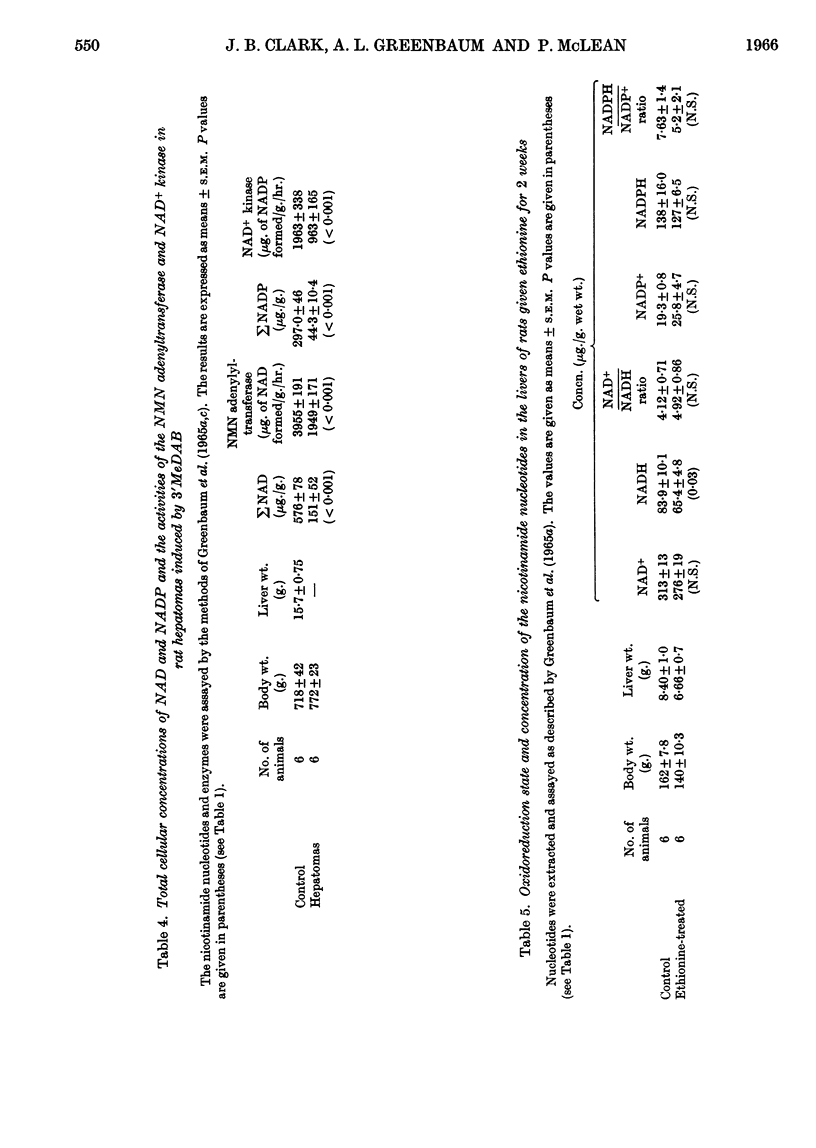
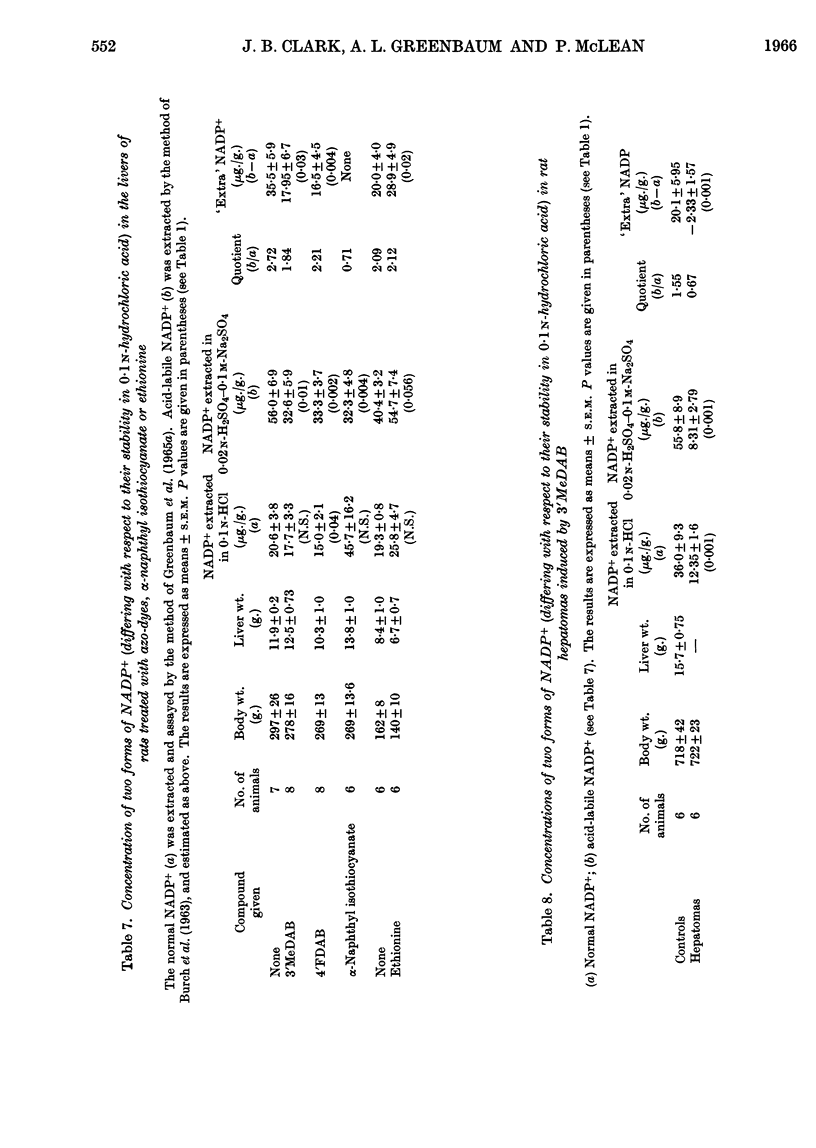
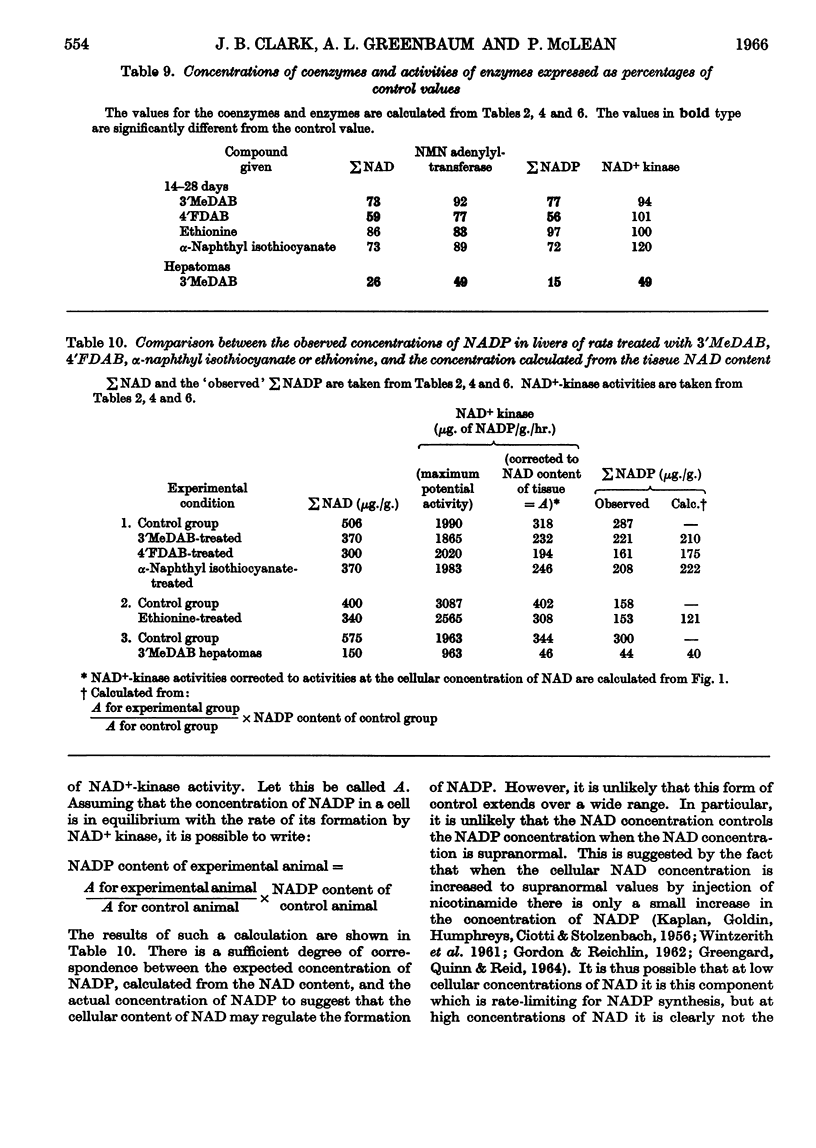
1. The oxidoreduction state and concentration of both NAD and NADP as well as the maximum potential activities of NMN adenylyltransferase and NAD+ kinase have been measured in the livers of rats treated for 14–28 days with 4-dimethylamino-3′-methylazobenzene, 4-dimethylamino-4′-fluoroazobenzene, α-naphthyl isothiocyanate or ethionine and in primary hepatomas induced by 4-dimethylamino-3′-methylazobenzene. 2. The total NAD and total NADP both decreased in the livers of rats treated with either azo-dyes or α-naphthyl isothiocyanate but not in those treated with ethionine. The activities of NMN adenylyltransferase and NAD+ kinase did not alter appreciably after such treatments. 3. In the primary hepatomas the concentrations of both NAD and NADP fell drastically and the activities of NMN adenylyltransferase and NAD+ kinase fell to about 50% of the control activities. 4. No correlation could be established between the concentrations of the nucleotides and the activities of the enzymes synthesizing them. It appears, however, that a relationship exists between the NAD content of the tissue and the amount of NADP present. 5. The results are discussed with respect to the control of NAD and NADP synthesis by ATP. At the concentrations of NAD normally present in the cell it is suggested that NAD may be a rate-limiting substrate in NADP synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., JACKSON J. F., MORTON R. K. Substrate specificity and inhibition of nicotinamide mononucleotideadenylyl transferase of liver nuclei: possible mechanism of effect of 6-mercaptopurine on tumour growth. Nature. 1961 Dec 9;192:946–948. doi: 10.1038/192946a0. [DOI] [PubMed] [Google Scholar]
- BRANSTER M. V., MORTON R. K. Comparative rates of synthesis of diphosphopyridine nucleotide by normal and tumour tissue from mouse mammary gland; studies with isolated nuclei. Biochem J. 1956 Aug;63(4):640–646. doi: 10.1042/bj0630640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGGS M. H. Vitamin and coenzyme content of hepatomas induced by buter yellow. Nature. 1960 Jul 16;187:249–250. doi: 10.1038/187249a0. [DOI] [PubMed] [Google Scholar]
- BRUCE H. M., PARKES A. S. Feeding and breeding of laboratory animals; a complete cubed diet for mice and rats. J Hyg (Lond) 1949 Jun;47(2):202–208. doi: 10.1017/s0022172400014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., VONDIPPE P. THE STABILITY OF TRIPHOSPHOPYRIDINE NUCLEOTIDE AND ITS REDUCED FORM IN RAT LIVER. J Biol Chem. 1963 Aug;238:2838–2842. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. Levels of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in tumours. Biochem J. 1957 Feb;65(2):413–416. doi: 10.1042/bj0650413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. The determination of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in animal tissues. Biochem J. 1955 Nov;61(3):381–388. doi: 10.1042/bj0610381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON E. E., REICHLIN M. The generation of reduced pyridine nucleotides in the liver of the intact rat. Biochim Biophys Acta. 1962 Feb 12;57:160–162. doi: 10.1016/0006-3002(62)91097-1. [DOI] [PubMed] [Google Scholar]
- GRANT H. C., REES K. R. The precancerous liver; correlations of histological and biochemical changes in rats during prolonged administration of thioacetamide and butter yellow. Proc R Soc Lond B Biol Sci. 1958 Jan 1;148(930):117–136. doi: 10.1098/rspb.1958.0010. [DOI] [PubMed] [Google Scholar]
- GREENBAUM A. L., CLARK J. B., MCLEAN P. THE ESTIMATION OF THE OXIDIZED AND REDUCED FORMS OF THE NICOTINAMIDE NUCLEOTIDES. Biochem J. 1965 Apr;95:161–166. doi: 10.1042/bj0950161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., CLARK J. B. THE EFFECT OF DIFFERENT HORMONAL CONDITIONS ON THE CONCENTRATION AND OXIDOREDUCTION STATE OF THE NICOTINAMIDE NUCLEOTIDES OF RAT LIVER. Biochem J. 1965 Apr;95:167–179. doi: 10.1042/bj0950167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., QUINN G. P., REID M. B. PITUITARY INFLUENCE OF PYRIDINE NUCLEOTIDE METABOLISM OF RAT LIVER. J Biol Chem. 1964 Jun;239:1887–1892. [PubMed] [Google Scholar]
- Greenbaum A. L., Clark J. B., McLean P. The activities of nicotinamide mononucleotide adenylyltransferase and of nicotinamide-adenine dinucleotide kinase in the livers of rats subjected to different hormonal treatments. Biochem J. 1965 Aug;96(2):507–516. doi: 10.1042/bj0960507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEDEIKIN L., THOMAS A. J., WEINHOUSE S. Metabolism of neoplastic tissue. X. Diphosphopyridine nucleotide levels during azo dye hepatocarcinogenesis. Cancer Res. 1956 Oct;16(9):867–872. [PubMed] [Google Scholar]
- KAPLAN N. O., GOLDIN A., HUMPHREYS S. R., CIOTTI M. M., STOLZENBACH F. E. Pyridine nucleotide synthesis in the mouse. J Biol Chem. 1956 Mar;219(1):287–298. [PubMed] [Google Scholar]
- KOTNIS L. B., NARURKAR M. V., SAHASRABUDHE M. B. Role of pyridine nucleotides and hexose monophosphate pathway in butter yellow (DAB) carcinogenesis. Br J Cancer. 1962 Sep;16:550–555. doi: 10.1038/bjc.1962.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON R. K. Enzymic synthesis of coenzyme I in relation to chemical control of cell growth. Nature. 1958 Feb 22;181(4608):540–542. doi: 10.1038/181540a0. [DOI] [PubMed] [Google Scholar]
- NODES J. T., REID E. AZO-DYE CARCINOGENESIS: RIBONUCLEOTIDES AND RIBONUCLEASES. Br J Cancer. 1963 Dec;17:745–774. doi: 10.1038/bjc.1963.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUASTEL J. H., ZATMAN L. J. Effects of extracts of normal tissues and of tumours on yeast fermentation; estimations of relative diphosphopyridine nucleosidase activities. Biochim Biophys Acta. 1953 Feb;10(2):256–267. doi: 10.1016/0006-3002(53)90249-2. [DOI] [PubMed] [Google Scholar]
- REID E. Significant biochemical effects of hepatocarcinogens in the rat: a review. Cancer Res. 1962 May;22:398–430. [PubMed] [Google Scholar]
- SHIMOYAMA M., KORI J., USUKI K., LAN S. J., GHOLSON R. K. ENZYMIC LESIONS OF NICOTINAMIDE-ADENINE DINUCLEOTIDE BIOSYNTHESIS IN HEPATOMAS. Biochim Biophys Acta. 1965 Feb 15;97:402–404. doi: 10.1016/0304-4165(65)90124-8. [DOI] [PubMed] [Google Scholar]
- STEKOL J. A., BEDRAK E., MODY U., BURNETTE N., SOMERVILLE C. Inhibition of the synthesis of diphosphopyridine nucleotide by ethionine administration in the liver of rats and mice. J Biol Chem. 1963 Jan;238:469–473. [PubMed] [Google Scholar]
- WINTZERITH M., KLEIN N., MANDEL L., MANDEL P. Comparison of pyridine nucleotides in the liver and in an ascitic hepatoma. Nature. 1961 Jul 29;191:467–469. doi: 10.1038/191467a0. [DOI] [PubMed] [Google Scholar]


