Abstract
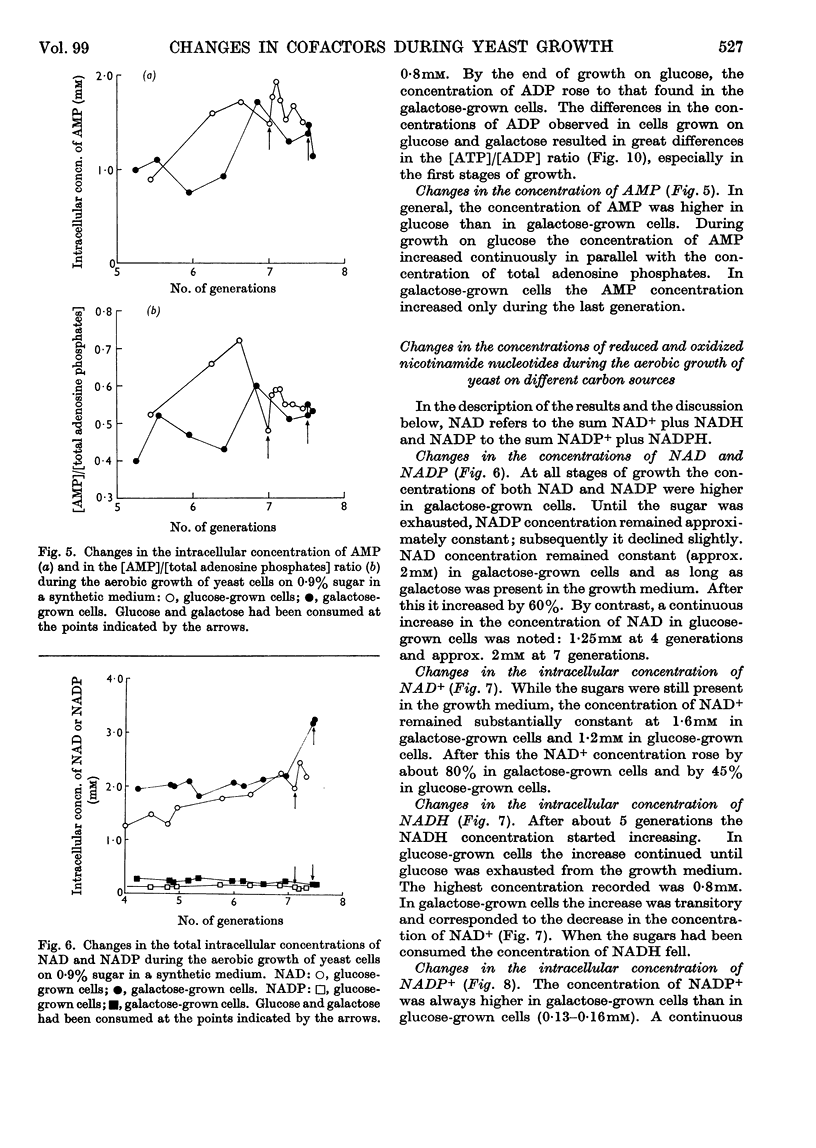
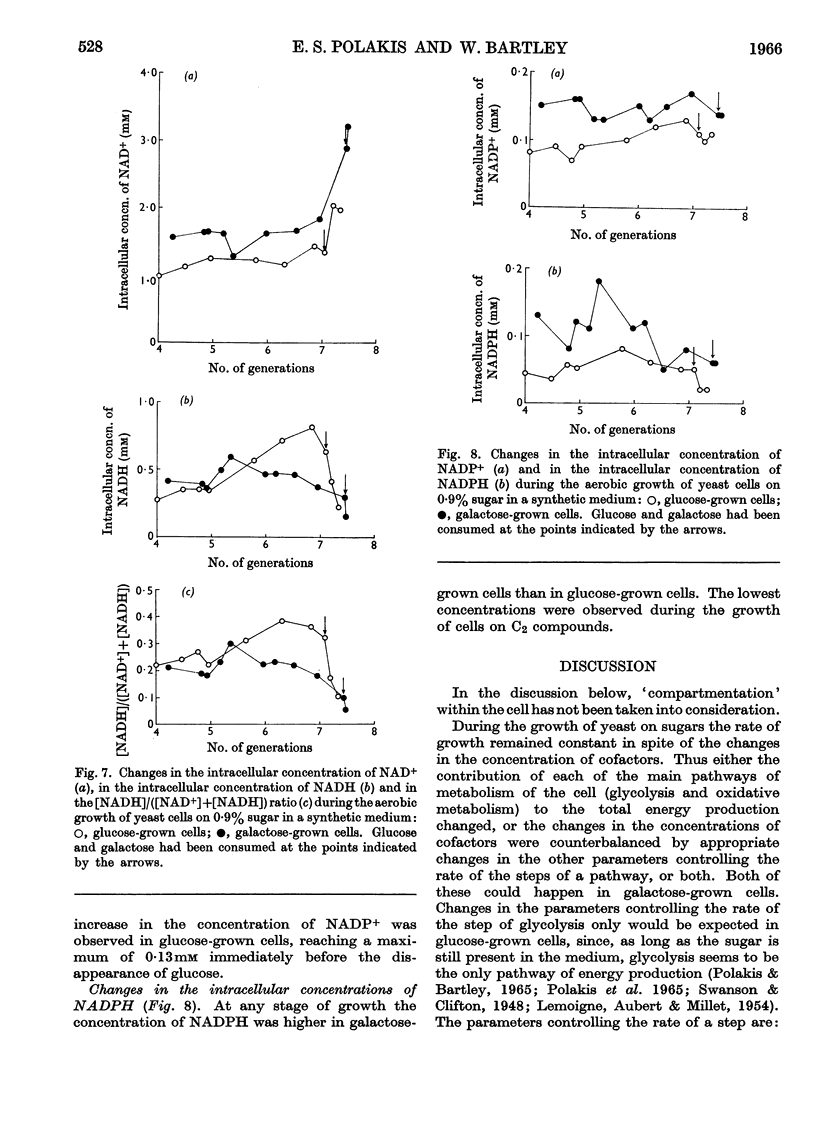
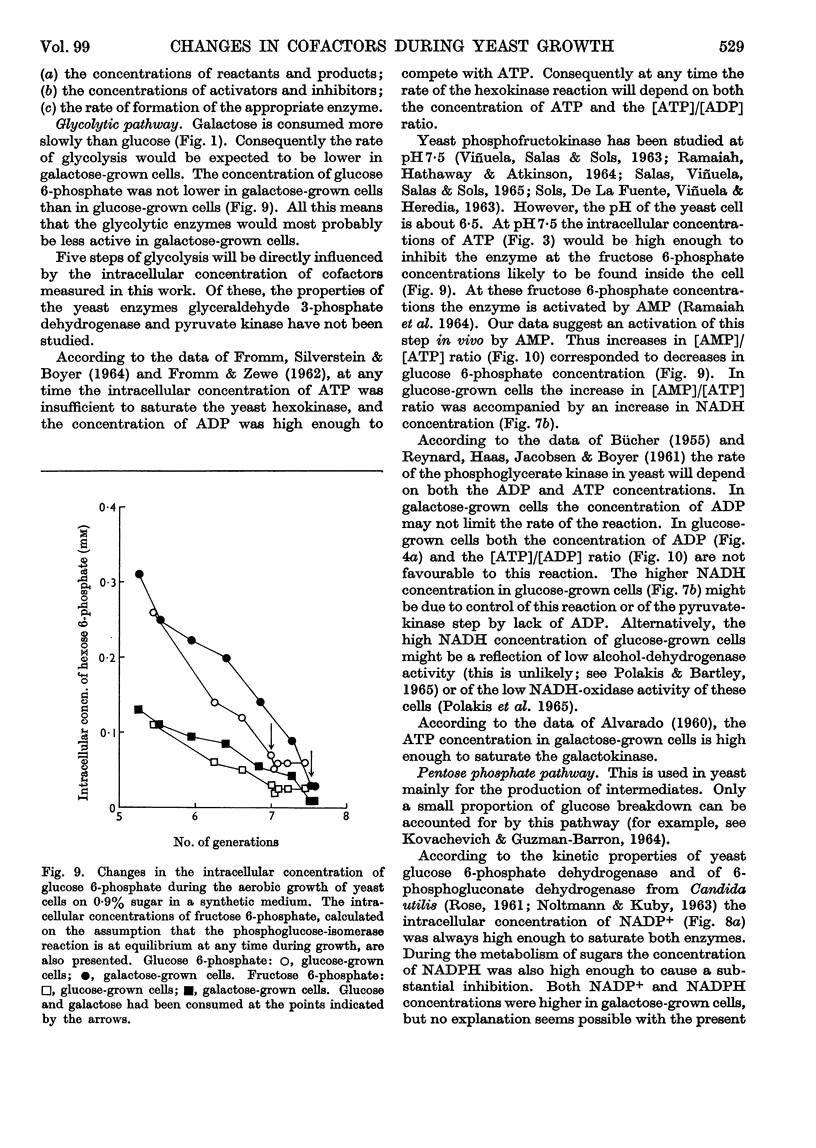
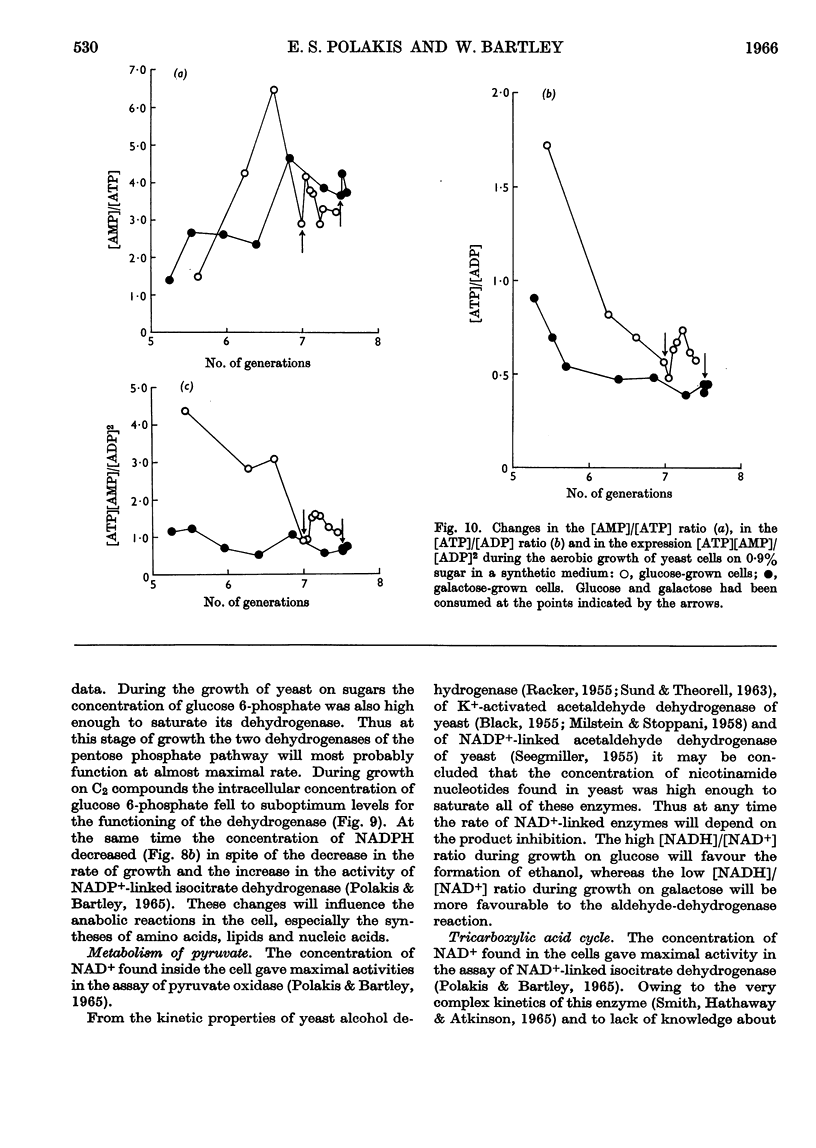
1. Methods for the quantitative extraction of adenosine phosphates and nicotinamide nucleotides from yeast cells are described. 2. The intracellular concentrations of adenosine phosphates and nicotinamide nucleotides were measured during the aerobic growth cycle of yeast on glucose and galactose. 3. When sugars were still present in the media the intracellular concentrations of NADH and AMP were in general higher in glucose- than in galactose-grown cells, whereas ADP concentration was always lower in glucose-grown cells. 4. The adenylate-kinase reaction was found to be far from equilibrium in the glucose-grown cells and when glucose was still present in the growth medium. 5. The significance of the changes in the intracellular concentrations of adenosine phosphates and nicotinamide nucleotides observed during growth on either sugar is discussed in relation to the metabolism and growth of the cells. 6. The differences observed in the concentrations of these cofactors in glucose- and galactose-grown cells are also discussed in relation to the type of metabolism of these cells. Control of glycolysis at the level of phosphofructokinase in galactose-grown cells and at the level of phosphoglycerate kinase in glucose-grown cells is suggested. 7. ADP is suggested to be the inducer of formation of respiratory enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO F. Substrate specificity of Saccharomyces fragilis galactokinase. Biochim Biophys Acta. 1960 Jul 1;41:233–238. doi: 10.1016/0006-3002(60)90005-6. [DOI] [PubMed] [Google Scholar]
- BOWEN W. J., KERWIN T. D. The kinetics of myokinase. II. Studies of heat denaturation, the effects of salts and the state of equilibrium. Arch Biochem Biophys. 1956 Oct;64(2):278–284. doi: 10.1016/0003-9861(56)90270-3. [DOI] [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., VONDIPPE P. THE STABILITY OF TRIPHOSPHOPYRIDINE NUCLEOTIDE AND ITS REDUCED FORM IN RAT LIVER. J Biol Chem. 1963 Aug;238:2838–2842. [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROMM H. J., SILVERSTEIN E., BOYER P. D. EQUILIBRIUM AND NET REACTION RATES IN RELATION TO THE MECHANISM OF YEAST HEXOKINASE. J Biol Chem. 1964 Nov;239:3645–3652. [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of yeast hexokinase. J Biol Chem. 1962 Oct;237:3027–3032. [PubMed] [Google Scholar]
- HATHAWAY J. A., ATKINSON D. E. THE EFFECT OF ADENYLIC ACID ON YEAST NICOTINAMIDE ADENINE DINUCLEOTIDE ISOCITRATE DEHYDROGENASE, A POSSIBLE METABOLIC CONTROL MECHANISM. J Biol Chem. 1963 Aug;238:2875–2881. [PubMed] [Google Scholar]
- HOLZER H., BUSCH D., KROGER H. Enzymatisch-optische Bestimmung von TPNH und TPN neben DPNH und DPN. Hoppe Seylers Z Physiol Chem. 1958;313:184–193. doi: 10.1515/bchm2.1958.313.1.184. [DOI] [PubMed] [Google Scholar]
- HOLZER H., HIERHOLZER G., WITT I. [alpha-Ketoglutarate oxidase of yeast]. Biochem Z. 1963;337:115–119. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KORNBERG A., PRICER W. E., Jr Di- and triphosphopyridine nucleotide isocitric dehydrogenases in yeast. J Biol Chem. 1951 Mar;189(1):123–136. [PubMed] [Google Scholar]
- KOVACHEVICH R., BARRON E. S. COMPARISONS OF THE PHOSPHOGLUCONATE OXIDATIVE PATHWAY IN NORMAL AND RESPIRATION-DEFICIENT MUTANT YEAST. Arch Biochem Biophys. 1964 Nov;108:200–206. doi: 10.1016/0003-9861(64)90376-5. [DOI] [PubMed] [Google Scholar]
- LEMOIGNE M., AUBERT J. P., MILLET J. La production d'alcool et le rendement de croissance de la levure de boulangerie cultivée en aérobiose. Ann Inst Pasteur (Paris) 1954 Oct;87(4):427–439. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., ROCK M. K. The stability of pyridine nucleotides. J Biol Chem. 1961 Oct;236:2756–2759. [PubMed] [Google Scholar]
- MILSTEIN C., STOPPANI A. O. Kinetics of the potassium-activated aldehyde dehydrogenase of yeast. Biochim Biophys Acta. 1958 Apr;28(1):218–219. doi: 10.1016/0006-3002(58)90460-8. [DOI] [PubMed] [Google Scholar]
- NEUBERT D., SCHULZ H. U., HOEHNE R. STABILITY OF NICOTINAMIDE-ADENINE DINUCLEOTIDE PHOSPHATE AND NICOTINAMIDE-ADENINE DINUCLEOTIDE IN TISSUE EXTRACTS UNDER MILDLY ACIDIC CONDITIONS. Biochim Biophys Acta. 1964 Dec 23;92:610–612. doi: 10.1016/0926-6569(64)90023-9. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in dry weight, protein, deoxyribonucleic acid, ribonucleic acid and reserve and structural carbohydrate during the aerobic growth cycle of yeast. Biochem J. 1966 Mar;98(3):883–887. doi: 10.1042/bj0980883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J. 1965 Oct;97(1):298–302. doi: 10.1042/bj0970298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAIAH A., HATHAWAY J. A., ATKINSON D. E. ADENYLATE AS A METABOLIC REGULATOR. EFFECT ON YEAST PHOSPHOFRUCTOKINASE KINETICS. J Biol Chem. 1964 Nov;239:3619–3622. [PubMed] [Google Scholar]
- REYNARD A. M., HASS L. F., JACOBSEN D. D., BOYER P. D. The correlation of reaction kinetics and substrate binding with the mechanism of pyruvate kinase. J Biol Chem. 1961 Aug;236:2277–2283. [PubMed] [Google Scholar]
- ROSE I. A. The use of kinetic isotope effects in the study of metabolic control. I. Degradation of glucose-1-D by the hexosemonophosphate pathway. J Biol Chem. 1961 Mar;236:603–609. [PubMed] [Google Scholar]
- SALAS M. L., VINUELA E., SALAS M., SOLS A. CITRATE INHIBITION OF PHOSPHOFRUCTOKINASE AND THE PASTEUR EFFECT. Biochem Biophys Res Commun. 1965 Apr 23;19:371–376. doi: 10.1016/0006-291x(65)90471-7. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Clifton C. E. Growth and Assimilation in Cultures of Saccharomyces cerevisiae. J Bacteriol. 1948 Jul;56(1):115–124. doi: 10.1128/jb.56.1.115-124.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINUELA E., SALAS M. L., SOLS A. End-product inhibition of yeast phosphofructokinase by ATP. Biochem Biophys Res Commun. 1963 Jul 18;12:140–145. doi: 10.1016/0006-291x(63)90250-x. [DOI] [PubMed] [Google Scholar]


