Abstract
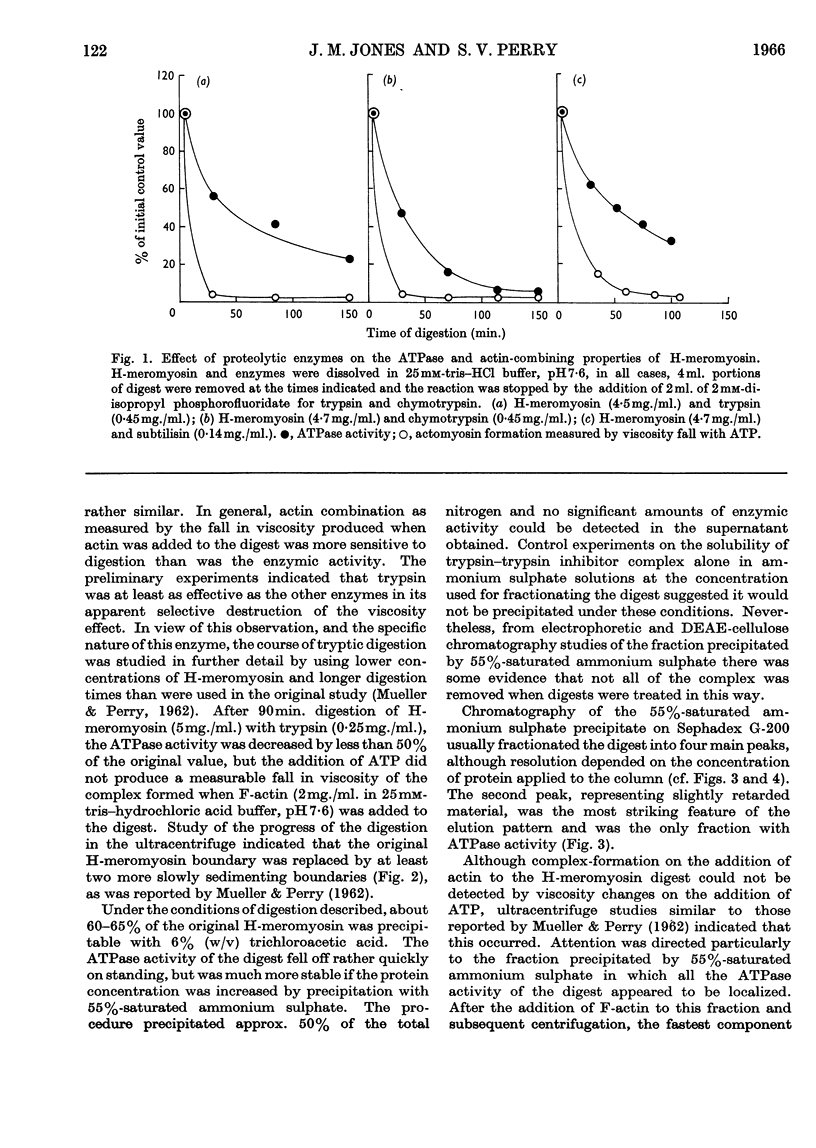
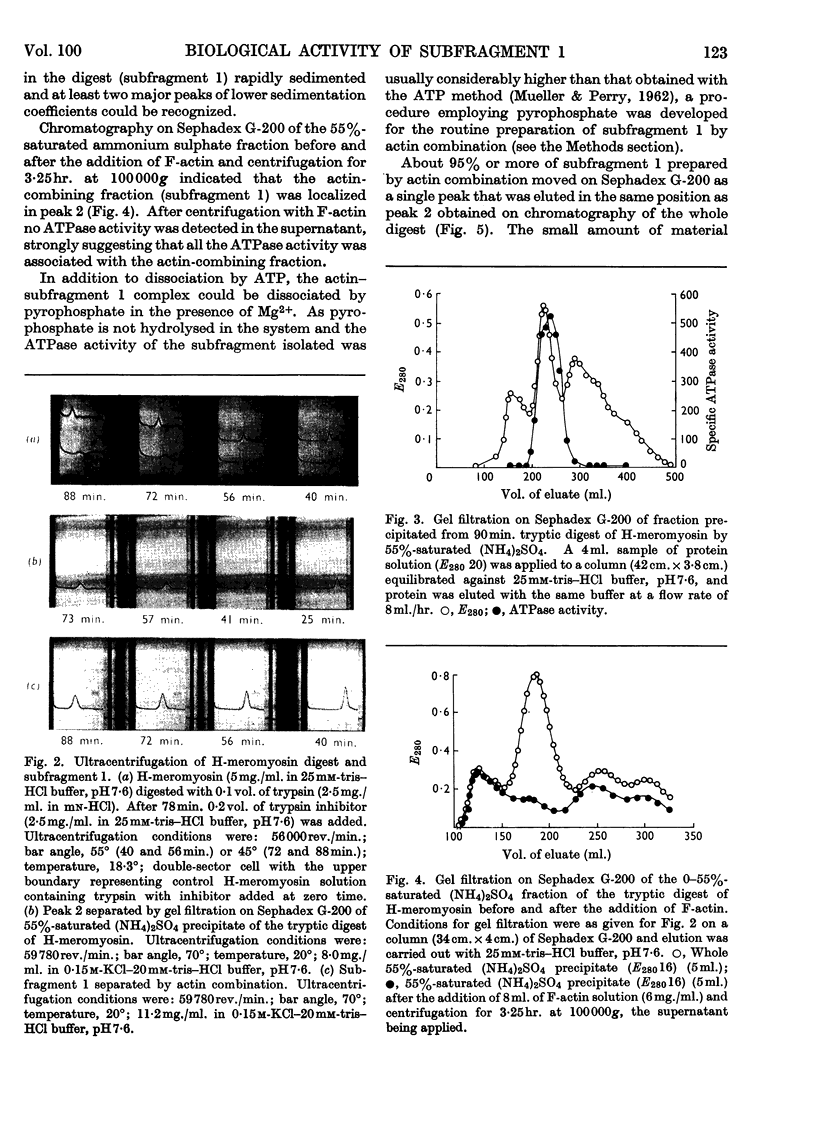
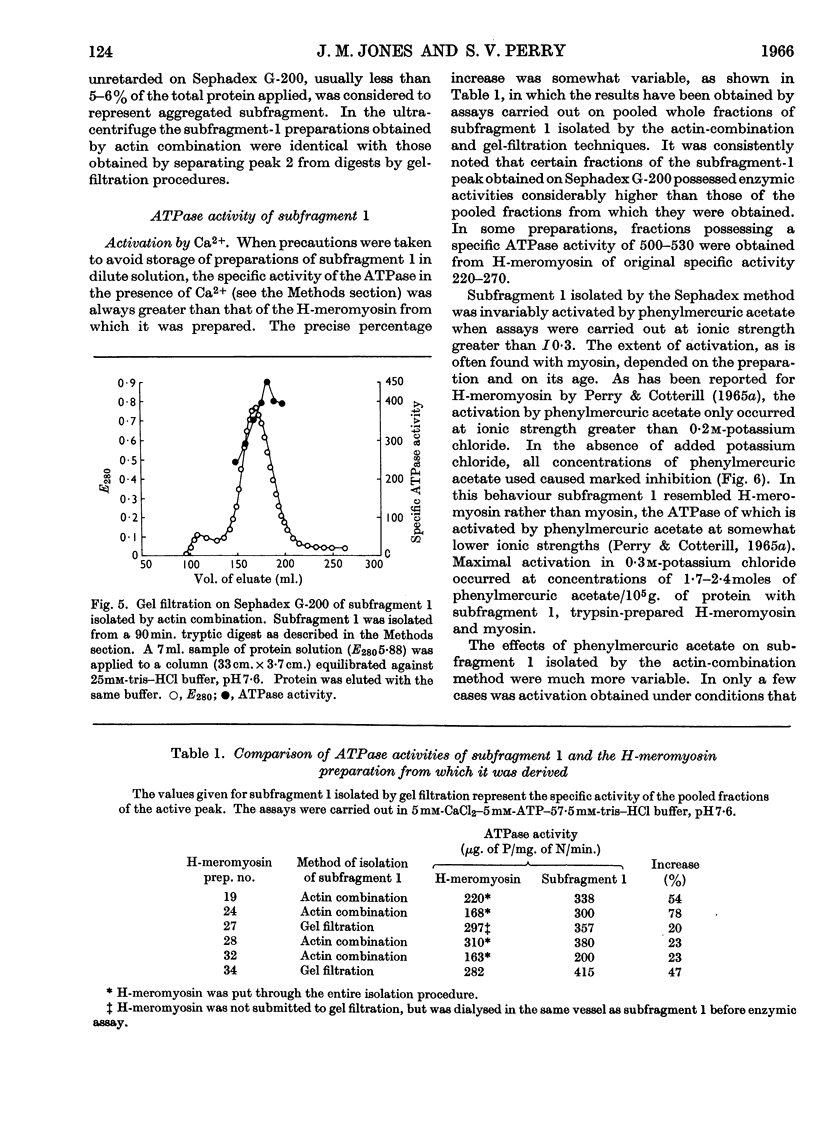
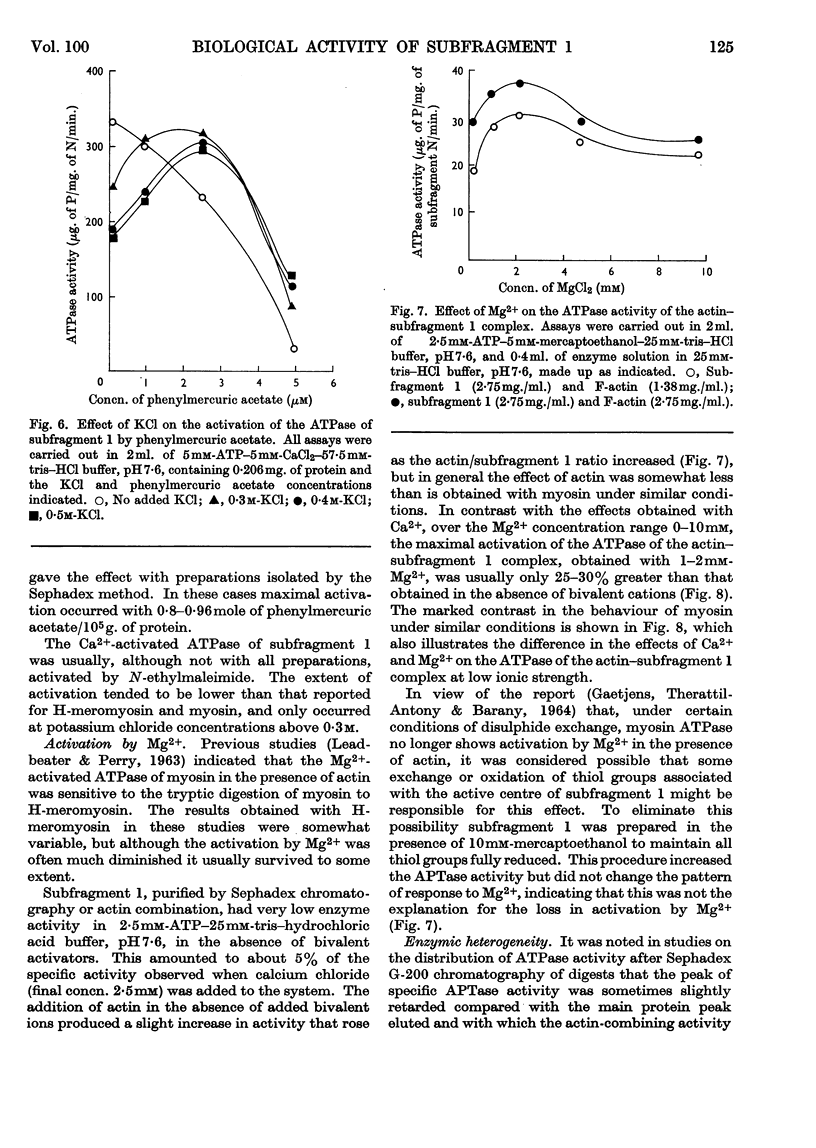
1. The action of trypsin, chymotrypsin and subtilisin on the adenosine-triphosphatase and actin-combining activities, as measured by viscometric means, of H-meromyosin were compared. 2. Subfragment 1 produced by prolonged tryptic digestion has a molecular weight of 129000. 3. The preparations isolated by gel filtration and actin combination were shown to be similar. 4. Subfragment-1 preparations possess appreciably higher adenosine-triphosphatase activities than H-meromyosin when related to total nitrogen. 5. Chromatographic and gelfiltration studies indicated that adenosine-triphosphatase activity is not distributed uniformly in all fractions of subfragment 1. 6. The Ca2+-activated adenosine triphosphatase of subfragment 1 was stimulated by thiol reagents in a similar fashion to myosin and H-meromyosin. 7. Subfragment 1 differed from myosin and H-meromyosin in that its adenosine triphosphatase was only slightly activated by Mg2+ in the presence of actin. 8. A subfragment-1-like component was obtained by chymotryptic digestion of H-meromyosin. 9. The results obtained from enzymic and hydrodynamic studies and from amino acid analyses are compatible with the concept of one molecule of H-meromyosin giving rise to one molecule of subfragment 1 on proteolytic digestion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANY M., BIRO N. A., MOLNAR J., STRAUB F. B. Darstellung enzymfreien Aktins durch Umfällung mit Magnesium. Acta Physiol Acad Sci Hung. 1954;5(3-4):369–381. [PubMed] [Google Scholar]
- BARGETZI J. P. KUMAR KS, COX DJ, WALSH KA, NEURATH H: THE AMINO ACID COMPOSITION OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. Biochemistry. 1963 Nov-Dec;2:1468–1474. doi: 10.1021/bi00906a046. [DOI] [PubMed] [Google Scholar]
- GAETJENS E., THERATTIL ANTONY T., BARANY M. MODIFICATION OF L-MYOSIN BY DISULFIDE-SULFHYDRYL INTERCHANGE REACTION. Biochim Biophys Acta. 1964 Jun 8;86:554–566. doi: 10.1016/0304-4165(64)90095-9. [DOI] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- LEADBEATER L., PERRY S. V. The effect of actin on the magnesium-activated adenosine triphosphatase of heavy meromyosin. Biochem J. 1963 May;87:233–239. doi: 10.1042/bj0870233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The chromatography of the meromyosins on diethylaminoethylcellulose. Biochem J. 1961 Jul;80:217–223. doi: 10.1042/bj0800217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- NANKINA V. P., KOFMAN E. B., CHERNIAK V. Ia, KALAMKAROVA M. B. PRODUKTY PROTEOLIZA TIAZHELOGO MEROMIOZINA, OBLADAIUSHCHIE ADENOZINTRIFOSFATAZNO I AKTIVNOST'IU. Biokhimiia. 1964 May-Jun;29:424–431. [PubMed] [Google Scholar]
- Nanninga L. B., Mommaerts W. F. STUDIES ON THE FORMATION OF AN ENZYME-SUBSTRATE COMPLEX BETWEEN MYOSIN AND ADENOSINETRIPHOSPHATE. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1155–1166. doi: 10.1073/pnas.46.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., COTTERILL J. THE ACTION OF THIOL REAGENTS ON THE ADENOSINE-TRIPHOSPHATASE ACTIVITIES OF HEAVY MEROMYOSIN AND L-MYOSIN. Biochem J. 1965 Jul;96:224–230. doi: 10.1042/bj0960224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The chromatography of L-myosin on diethylaminoethylcellulose. Biochem J. 1960 Jan;74:94–101. doi: 10.1042/bj0740094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. Interaction of actin and myosin. Nature. 1965 Apr 10;206(980):161–163. doi: 10.1038/206161a0. [DOI] [PubMed] [Google Scholar]
- RICE R. V. Conformation of individual macromolecular particles from myosin solution. Biochim Biophys Acta. 1961 Sep 30;52:602–604. doi: 10.1016/0006-3002(61)90427-9. [DOI] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. Meromyosins, the subunits of myosin. Arch Biochem Biophys. 1953 Feb;42(2):305–320. doi: 10.1016/0003-9861(53)90360-9. [DOI] [PubMed] [Google Scholar]
- WOODS E. F., HIMMELFARB S., HARRINGTON W. F. Studies on the structure of myosin in solution. J Biol Chem. 1963 Jul;238:2374–2385. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]



