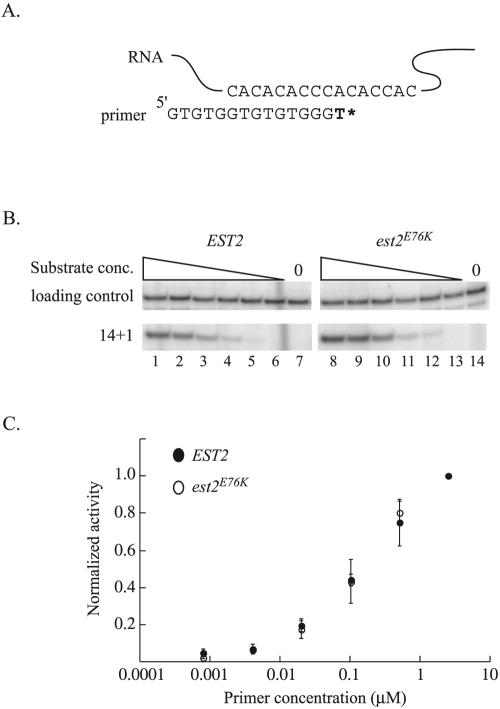
FIG. 4.
The ability of telomerase to bind primer is unchanged by mutation of Est2p residue E76. (A) Schematic diagram of the in vitro telomerase assay. Extension of a 14-nucleotide telomeric primer by immunopurified telomerase in the presence of radiolabeled TTP alone results in the addition of a single nucleotide. (B) Change in activity of wild-type and mutant telomerase with decreasing primer concentrations. Telomerase was partially purified from strain YKF120 (est2::HIS3) containing plasmids expressing either wild-type, protein A-tagged EST2 (ProA-EST2, lanes 1 to 7) or protein A-tagged est2E76K (ProA-est2E76K, lanes 8 to 14) by incubation with IgG-conjugated beads. Standard telomerase assays were conducted (see Materials and Methods) at decreasing primer concentrations. Production of the 14 + 1 product and recovery of a labeled 29-nucleotide precipitation and loading control is shown. Primer concentrations are 2.5 μM (lanes 1 and 8), 0.50 μM (lanes 2 and 9), 0.10 μM (lanes 3 and 10), 0.02 μM (lanes 4 and 11), 0.004 μM (lanes 5 and 12), and 0.0008 μM (lanes 6 and 13). Lanes 7 and 14 are assays done in the absence of primer. (C) Quantification of telomerase activity in response to decreasing primer concentrations. Band intensities in the experiment shown in Fig. 5B and others (data not shown) were determined by a phosphorimager. After adjustment for loading and precipitation efficiency, values were normalized to activity obtained at the highest primer concentration (see Materials and Methods for detail). Primer concentration (log axis) is graphed versus average, normalized activity level. Error bars represent standard deviations calculated from three independent experiments for the wild type and two independent experiments for est2E76K.