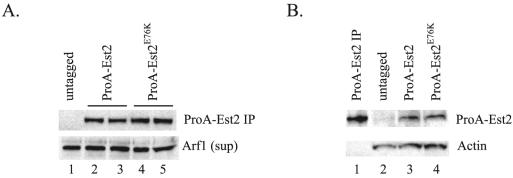
FIG. 5.
Protein expression of wild-type and mutant Est2p is equivalent. (A) Immunoprecipitation of ProA-Est2p from extract. ProA-Est2p was immunoprecipitated (IP) on IgG-conjugated beads from strain YKF120 (est2::HIS3) containing plasmids expressing either untagged EST2 (pKF404, lane 1), protein A-tagged EST2 (pKF410, lanes 2 and 3), or protein A-tagged est2E76K (pKF410-Est2E76K, lanes 3 and 4) and detected by Western blotting using an anti-protein A primary antibody (top band). Duplicate lanes (2 and 3; 4 and 5) represent two independent immunoprecipitations from the same extract preparation to show reproducibility. Supernatants (sup) from the immunoprecipitations were Western blotted for the presence of Arf1p to verify equivalent extract concentrations (bottom band). (B) Detection of ProA-Est2p in extract. Extracts from strain YKF120 (est2::HIS3) containing plasmids expressing either untagged EST2 (pKF404, lane 2), protein A-tagged EST2 (pKF410, lane 3), or protein A-tagged est2E76K (pKF410-Est2E76K, lane 4) were Western blotted and probed with an anti-protein A primary antibody (top band). Lane 1 contains immunoprecipitated ProA-Est2p (identical to panel A, lane 2) as a size marker. Actin was detected as a loading control (bottom band).