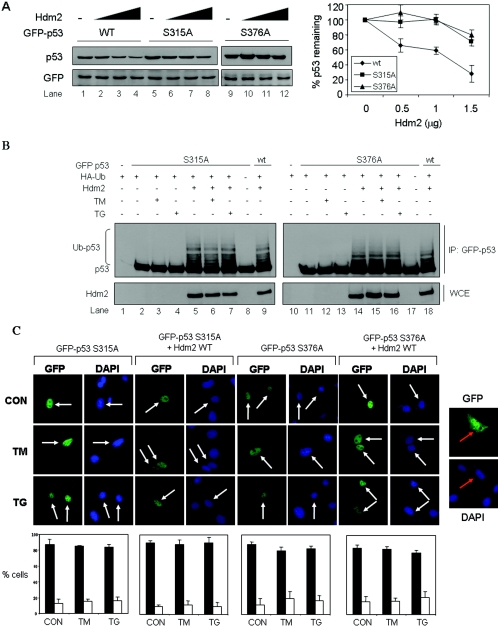
FIG. 4.
Role of S315 and S376 phosphorylation in subcellular localization and degradation of p53 in ER-stressed cells. (A) Hdm2-dependent degradation of GFP-p53 and its phosphorylation mutants. GFP-p53 WT cDNA or the indicated GFP-p53 mutant cDNAs (0.5 μg) were transiently transfected in 2KO cells in the presence of increasing amounts of Hdm2 WT cDNA (0.5 to 1.5 μg). The GFP cDNA (0.1 μg) was used as an internal control. After 24 h, protein extracts (50 μg) were used for immunoblotting with anti-p53 (DO-1) Ab (top panel) or anti-GFP Ab (bottom panel). The GFP-p53 proteins were normalized to GFP, and their degradation profiles with increasing amounts of Hdm2 are shown. The values in the graphs represent means ± the SD from three independent experiments. (B) Control of ubiquitylation of GFP-p53 phosphorylation mutants by Hdm2 in ER-stressed cells. GFP-p53 cDNA (0.5 μg) bearing the S315A or S376A mutation was transiently transfected in 2KO cells in the absence or presence of Hdm2 WT cDNA (0.5 μg) and HA-Ub cDNA (0.5 μg). The cells were treated with 10 μM MG132 for 3 h, followed by treatment with 10 μg of TM/ml or 1 μM TG for 3 h. The immunoprecipitation of GFP-p53 and immunoblotting were performed as in Fig. 3B. (C) Control of localization of GFP-p53 phosphorylation mutants by Hdm2. GFP-p53 mutant cDNAs (0.25 μg) were transiently transfected in 2KO cells in the absence or presence of Hdm2 WT cDNA (0.25 μg). After 24 h, cells were left untreated or treated with either 10 μg of TM/ml or 1 μM TG for 4 h, followed by the examination of GFP-p53 localization by fluorescence. Cell nuclei were visualized by staining with DAPI. Transfection of GFP cDNA alone was used as a control (right panel). White arrows indicate nuclear localization of GFP-p53 only. Quantification of GFP-p53 localization was performed as described for Fig. 2A and in Materials and Methods.