FIG. 6.
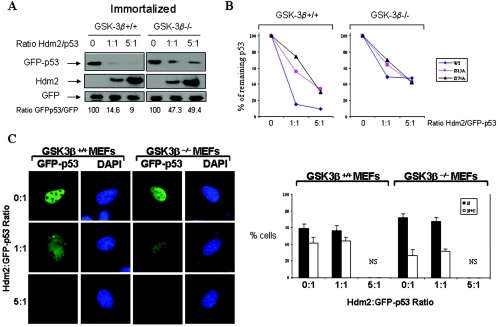
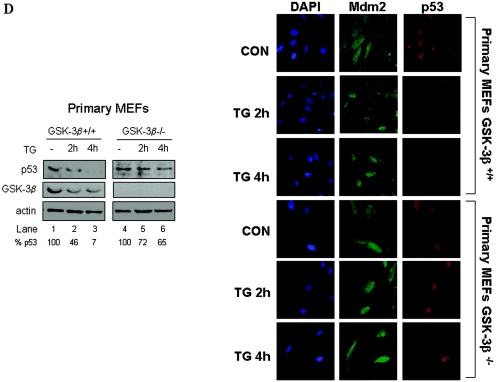
GSK-3β is necessary for p53 degradation in ER-stressed cells. (A) Control of GFP-p53 stability by GSK-3β. GFP-p53 WT cDNA (0.25 μg) was transiently transfected with increasing amounts of Hdm2 cDNA (0.25 to 1.25 μg) into immortalized GSK-3β−/− and GSK-3β+/+ MEFs. The GFP cDNA (0.25 μg) was used as an internal control. The GFP-p53, Hdm2, and GFP protein levels were detected by immunoblotting of 50 μg of protein extracts with antibodies against each protein. The bands were quantified with the Scion Image 2.0 software, and the ratio of GFP-p53 to GFP in each lane is indicated. (B) Hdm2-dependent degradation of GFP-p53 and its phosphorylation mutants. GFP-p53 WT cDNA or the indicated GFP-p53 phosphorylation mutants were cotransfected with Hdm2 WT cDNAs in immortalized GSK-3β−/− and GSK-3β+/+ MEFs as described in panel A. Immunoblot analyses of GFP-p53 and GFP were performed as in panel A. The bands were quantified, and the graphs representing the degradation rates of the GFP-p53 forms plotted against Hdm2/GFP-p53 ratios are shown. (C) Immortalized GSK-3β+/+ and GSK-3β−/− MEFs were transfected with different ratios of GFP-p53 WT to Hdm2 cDNAs as indicated. At 24 h posttransfection, cells were examined for GFP-p53 fluorescence. Cell nuclei were visualized by DAPI staining. The quantification of the subcellular distribution of GFP-p53 was performed as described for Fig. 2A and Materials and Methods. NS, not significant. (D) Regulation of p53 in primary GSK-3β−/− MEFs. MEFs (passage 3) were treated with 1 μM TG for 2 and 4 h, followed by immunoblotting of 50 μg of protein extracts for endogenous p53 (top panel), GSK-3β (middle panel), and actin (bottom panel). Immunostaining of endogenous Mdm2 (green) or p53 (red) in the primary MEFs was performed as described in Materials and Methods. The cell nuclei were detected by DAPI staining.