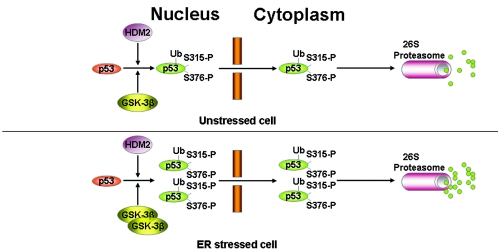
FIG. 7.
Schematic model of p53 regulation by Hdm2 in unstressed and ER-stressed cells. In unstressed cells, the nucleocytoplasmic transport of p53 is mainly mediated by Hdm2. We show here that phosphorylation of p53 at S315 and S376 by GSK-3β does not interfere with the ubiquitylation process but is required for the cytoplasmic relocation of p53 by Hdm2. Cytoplasmic p53 is then prone to degradation by the 26S proteasome pathway. When cells are exposed to ER stress, the nuclear fraction of GSK-3β becomes activated leading to the induction of p53 phosphorylation at S315 and S376 (49). As shown in the present study, these phosphorylation events enhance the ubiquitylation of p53 by Hdm2 and the cytoplasmic relocation of the tumor suppressor. As a result, p53 degradation rates in the cytoplasm are induced.