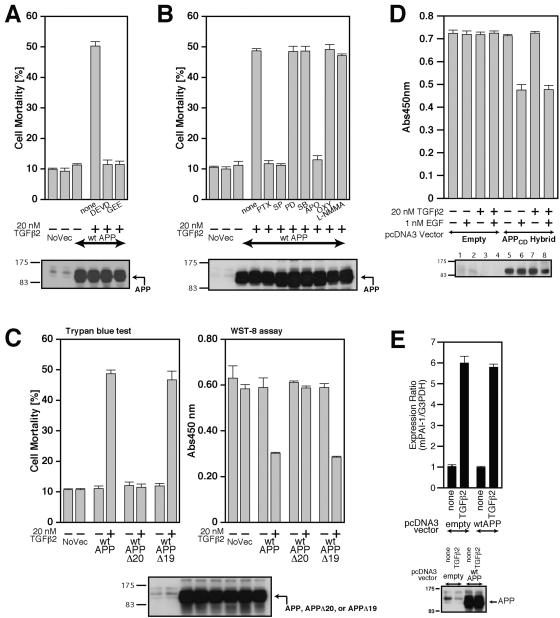
FIG. 7.
Characterization of TGFβ2-triggered death. (A and B) F11 cells, seeded on 6-well plates at 7 × 104 cells/well, were transfected with 0.5 μg of pcDNA3-wtAPP and then treated with 20 nM TGFβ2 in the presence or the absence of either 100 μM Ac-DEVD-CHO (DEVD), 1 mM GEE (A), 1 μg/ml PTX, 1 μM SP600125 (SP), 50 μM PD98059 (PD), 20 μM SB203580 (SB), 300 μM apocynin (APO), 100 μM oxypurinol (OXY), or 1 mM L-NMMA (B). Cell lysates (20 μg in each lane) were submitted to immunoblot analysis with 22C11 for APP. (C) F11 cells, seeded on 6-well plates at 7 × 104 cells/well, were transfected with 0.5 μg of pcDNA3-wtAPP, pcDNA3-wtAPPΔ20, or pcDNA3-wtAPPΔ19 and then treated with or without 20 nM TGFβ2. Cell mortality (trypan blue exclusion assay) and cell viability (WST-8 assay) were determined at 48 h. (D) TGFβ2 treatment does not enhance EGFR-ED+TM/APP-CD (labeled APPCD-Hybrid)-mediated death of F11 cells induced by treatment with 1 nM EGF. F11 cells, transfected with 1.0 μg of pcDNA3-EGFR-ED+TM/APP-CD or the backbone pcDNA3 vector, were treated with 1 nM EGF together with or without 20 nM TGFβ2. Cell viability was determined by WST-8 assays at 48 h after the onset of TGFβ2 treatment. Cell lysates (20 μg in each lane) were immunoblotted with antibody to EGFR to detect the APPCD hybrid. (E) Enforced expression of wtAPP did not result in the enhancement of the TGFβ2-induced activation of plasminogen activator inhibitor-1 (PAI-1) mRNA expression. F11 cells, transfected with 0.5 μg of the pcDNA3 vector or pcDNA3-wtAPP, were treated with 100 pM of TGFβ2 at 24 h after transfection. After incubation for 48 h, they were harvested for real-time PCR-based determination of mRNA amounts. NoVec, no vector; Abs450nm, absorbance at 450 nm; mPAI-1, mouse PAI-1.