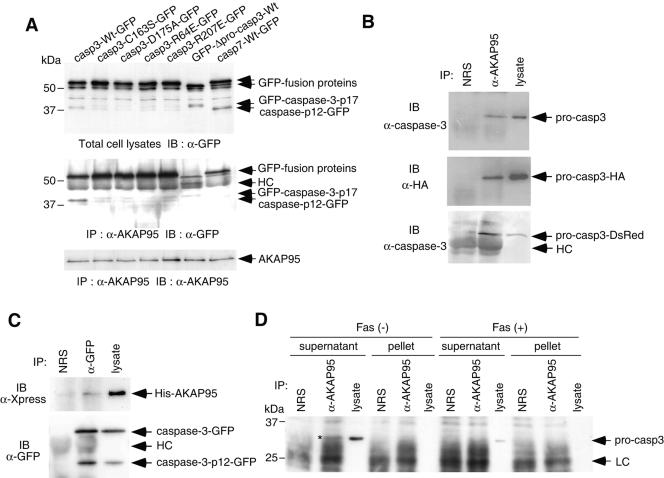
FIG. 3.
In vivo association of caspase 3 with AKAP95. (A) Active caspase 3, but not active caspase-7, binds to AKAP95. 293T cells were transfected with caspase-GFP expression plasmids as indicated, together with pCAG-AKAP95-Wt. Casp3-C163S-GFP contained the mutation at the catalytic Cys, casp3-D175A-GFP contained the cleavage site mutation between the p17 and p12 subunits, casp3-R64E-GFP and casp3-R207E-GFP contained the mutations of substrate recognition sites, and GFP-Δpro-casp3-Wt was a prodomain deletion mutant. After incubation for 24 h, lysates were immunoprecipitated with anti-AKAP95 serum. The input lysates (upper panel) and the immunoprecipitates (middle and lower panels) were fractionated by SDS-PAGE and immunoblotted with anti-GFP monoclonal antibody (upper and middle panels) or anti-AKAP95 monoclonal antibody (lower panel). HC, heavy chain. (B) Coprecipitation of procaspase 3 with AKAP95 in transiently overexpressed 293T cells. Lysates from 293T cells transfected with either pCAG-casp3, pCAG-casp3-HA, or pcasp3-Wt-DsRed, together with pCAG-AKAP95-Wt, were immunoprecipitated with normal rabbit serum (NRS) or anti-AKAP95 serum (α-AKAP95). The immunoprecipitates and the input lysates were fractionated by SDS-PAGE and immunoblotted with anti-caspase 3 monoclonal antibody (upper and lower panels) or anti-HA monoclonal antibody (12CA5) (middle panel). (C) Coprecipitation of AKAP95 with caspase 3 in transiently overexpressed 293T cells. Lysates from 293T cells transfected pcDNA-AKAP95-Wt together with pcasp3-Wt-GFP were immunoprecipitated with normal rabbit serum (NRS) or anti-GFP polyclonal antibodies (α-GFP). The immunoprecipitates and the input lysates were fractionated by SDS-PAGE and immunoblotted with anti-Xpress monoclonal antibody that recognizes the leader peptide from the pcDNA3.1/His vector between His tag and AKAP95 (upper panel) or anti-GFP monoclonal antibody (lower panel). (D) Procaspase 3 binds to AKAP95 at endogenous protein levels. HepG2 cells treated with or without an agonistic anti-Fas antibody in the presence of actinomycin D for 12 h were divided into supernatant or pellet fractions after lysis with digitonin and immunoprecipitated as described in panel B. The immunoprecipitates and the input lysates were fractionated by SDS-PAGE and immunoblotted with anti-caspase 3 monoclonal antibody. The asterisk indicates the procaspase 3 coprecipitated with AKAP95. LC, light chain.