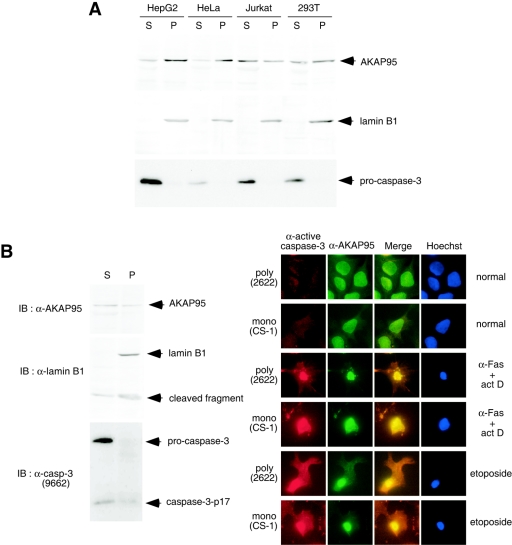
FIG. 4.
Localization of caspase 3 and AKAP95. (A) Localization of AKAP95 and procaspase 3 in normal cells. HepG2, HeLa, Jurkat, and 293T cells were harvested and fractionated into pellet (P) and supernatant (S) fractions after lysis with digitonin. Each fraction was subjected to SDS-PAGE and immunoblotted with anti-AKAP95 monoclonal antibody (upper panel), anti-lamin B1 polyclonal antibodies as a nuclear fraction marker (middle panel), or anti-caspase 3 monoclonal antibody (lower panel). (B) Colocalization of active caspase 3 and AKAP95 in apoptotic cells. HepG2 cells treated with an agonistic anti-Fas antibody in the presence of actinomycin D for 12 h were fractionated as described for panel A. Each fraction was subjected to SDS-PAGE and immunoblotted with anti-AKAP95 monoclonal antibody, anti-lamin B1 polyclonal antibodies, or anti-caspase 3 polyclonal antibodies (antibody 9662; Cell Signaling Technology) that detect both procaspase 3 and caspase 3-p17 as indicated (left panel). HepG2 cells were treated without or with an agonistic anti-Fas antibody in the presence of actinomycin D for 12 h or with etoposide for 40 h. After fixation and permeabilization, the cells were incubated with anti-active caspase 3 polyclonal (antibody 2622) or monoclonal (CS-1) antibodies, with anti-AKAP95 monoclonal or polyclonal antibodies, and with Texas Red (TXRD)- or fluorescein isothiocyanate-labeled secondary antibodies, followed by staining the nuclei with Hoechst 33342 (right panel).