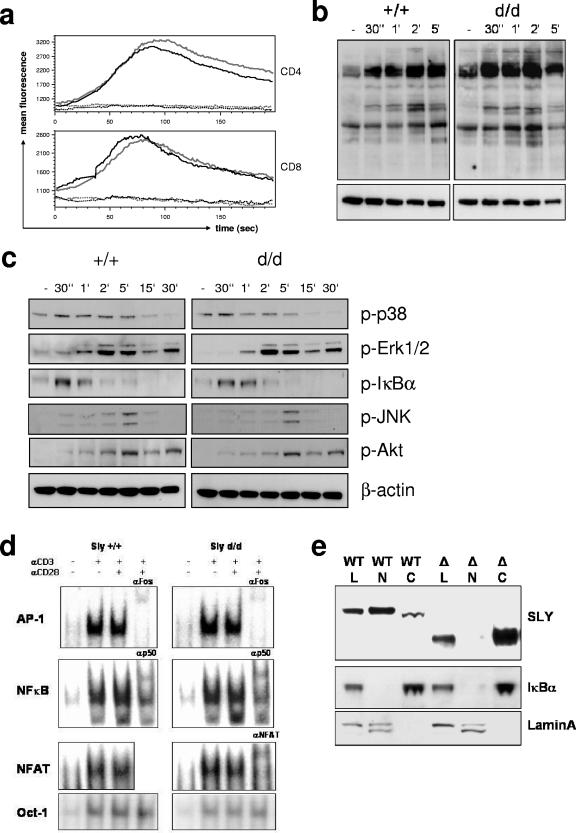
FIG. 7.
Influence of the Sly1d/d mutation on T-cell receptor signaling and subcellular localization of SLY1 protein. (a) Splenocytes were loaded with Fluo-4 and stained for CD4 and CD8 on ice. After warming, baseline fluorescence was determined and T-cell receptor signaling was induced by CD3 cross-linking. (b) T cells were stimulated with anti-CD3 for the indicated periods. Cell lysates were analyzed by Western blotting with antibodies against phosphotyrosine, as well as (c) phospho-p38, phospho-Erk1/2, phospho-JNK, phospho-Akt, and phospho-IκBα as indicated. Blots were stripped and reprobed with anti-β-actin as a loading control. (d) T cells were stimulated for 16 h as indicated and mobility shift assays with specific oligonucleotides were performed. Antibodies used for supershifts are indicated. (e) For subcellular localization of SLY1 protein, whole-cell lysates (L) as well as cytoplasmic (C) and nuclear (N) fractions from lymphocytes were prepared, followed by Western blot analysis with anti-SLY1 (2-16) antibody. The blots were stripped and reprobed with anti-IκBα and anti-lamin A/C antibodies to control for the purity of the preparations.