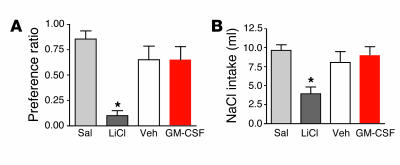
Figure 3.
Tests for visceral illness following injection of GM-CSF. (A) Injection of isotonic saline (Sal) or lithium chloride (LiCl) or an i3vt injection of 0.6 μg GM-CSF or vehicle was paired with introduction of a novel grape- or cherry-flavored 0.15% saccharin solution. On a subsequent test day, intake of the flavor paired with LiCl was reduced, indicating development of a CTA. The preference for the flavor paired with GM-CSF injection did not differ from that of vehicle or saline, demonstrating that GM-CSF did not support a CTA. *P < 0.05; n = 7–9; mean ± SEM. (B) Sodium-depleted rats were given an i.p. injection of isotonic saline or LiCl or an i3vt injection of 0.6 μg GM-CSF or vehicle. Cumulative sodium solution (NaCl) intake was measured at 2 hours. In contrast to LiCl-injected rats, cumulative intake of NaCl was not suppressed in rats receiving GM-CSF treatment as compared with control rats receiving saline or vehicle. *P < 0.05; n = 7–9; mean ± SEM.