Abstract
The mechanism(s) underlying the antibacterial activity of probiotic Lactobacillus strains appears to be multifactorial and includes lowering of the pH and the production of lactic acid and of antibacterial compounds, including bacteriocins and nonbacteriocin, non-lactic acid molecules. Addition of Dulbecco's modified Eagle's minimum essential medium to the incubating medium delays the killing activity of lactic acid. We found that the probiotic strains Lactobacillus johnsonii La1, Lactobacillus rhamnosus GG, Lactobacillus casei Shirota YIT9029, L. casei DN-114 001, and L. rhamnosus GR1 induced a dramatic decrease in the viability of Salmonella enterica serovar Typhimurium SL1344 mainly attributable to non-lactic acid molecule(s) present in the cell-free culture supernatant (CFCS). These molecules were more active against serovar Typhimurium SL1344 in the exponential growth phase than in the stationary growth phase. We also showed that the production of the non-lactic acid substance(s) responsible for the killing activity was dependent on growth temperature and that both unstable and stable substances with killing activity were present in the CFCSs. We found that the complete inhibition of serovar Typhimurium SL1344 growth results from a pH-lowering effect.
Probiotic strains are defined as live microorganisms which, when consumed in appropriate amounts in the food, confer a health benefit on the host (7). The probiotic strains that are currently most often being investigated are Lactobacillus spp. and bifidobacteria. The effectiveness of selected Lactobacillus strains used as probiotics to prevent and treat infectious bacterial and viral diarrhea, Helicobacter pylori gastroenteritis, and urovaginal infections has been demonstrated in well-designed in vitro and in vivo experimental studies and double-blind, placebo-controlled clinical trials (23, 24). It is important to note that previous reports have clearly indicated that the antibacterial activity against bacterial pathogens is a strain-specific property and cannot be extrapolated to other Lactobacillus strains.
The mechanism(s) of the antibacterial activity of probiotic Lactobacillus strains appears to be multifactorial (24). In particular, by producing metabolites such as acetic and lactic acid and thus lowering the pH, Lactobacillus strains inhibit the growth of bacterial pathogens and sometimes even kill them (28). The anti-Salmonella enterica serovar Typhimurium killing activity of probiotic Lactobacillus and Bifidobacterium strains has been previously investigated using an in vitro method in which the activity was measured in the presence of Luria broth (LB) or phosphate-buffered saline (PBS) (2, 5, 15). Using a new in vitro method, we were able to distinguish between the lactic acid- and non-lactic acid-dependent anti-Salmonella activities of Lactobacillus strains known to be probiotic.
MATERIALS AND METHODS
Bacterial strains.
Salmonella enterica serovar Typhimurium strain SL 1344 (S. enterica serovar Typhimurium SL1344) (6) was from Stocker B.A.D. (Stanford, California). Bacteria were grown for 24 h at 37°C in LB (Invitrogen, Cergy, France). Bacteria were subcultured in LB at 37°C and used until they reached the early logarithmic phase of growth.
Lactobacillus johnsonii strain La1 was from the Nestec Research Center at Vers-chez-les-Blanc, Switzerland (2). L. rhamnosus strain GG was a gift from S. L. Gorbach (Tufts University). L. rhamnosus strain GR1 was a gift from G. Reid (Lawson Health Research Institute, Canadian Research and Development Center for Probiotics, London, Ontario, Canada). L. casei Shirota strain YT9029 was from Yakult Honsha Co., Ltd. (Japan). L. casei strain DN-114 001 was from Danone Research Center (Le Plessis-Robinson, France). The food Lactobacillus strain L. sakei CWBI 030202 was from the Centre Wallon de Biologie Industrielle (Université de Liège, Sart-Tilman, Belgium).
The Lactobacillus strains were grown aerobically in 10 ml of De Man, Rogosa, Sharpe (MRS) broth (Difco Laboratories, Detroit, MI) 24 h at 37°C. Cell-free culture supernatants (CFCSs) were obtained by centrifuging at 10,000 × g for 30 min at 4°C. Centrifuged CFCSs were passed through a sterile 0.22-μm-pore-size Millex GS filter unit (Millipore, Molsheim, France). Since pH and the presence of metabolites such as lactic acid have been shown to inhibit the growth of pathogens and even kill them (28), three controls were used in these experiments. Since the different CFCSs exhibited pH values ranging from 3.9 to 4.5, all the Lactobacillus CFCSs were adjusted to pH 4.5 in all experiments. In some experiments, Lactobacillus CFCSs were adjusted to pH 6.5 with NaOH. Fresh MRS broth adjusted to pH 4.5 with HCl (MRS-HCl) was used as the first control. It is noteworthy that the probiotic Lactobacillus strains all produce l-lactic acid while L. sakei forms d,l-lactic acid. MRS broth containing d,l-lactic acid (60 mM; MRS-LA) as indicated was used as the second control. Finally, MRS broth adjusted to pH 6.5 with NaOH was used as the third control.
Determination of the lactic acid concentration.
A commercial d- and l-lactic acid determination kit of was used to determine the concentration of lactic acid in the CFCSs (Test-Combination d-lactic acid/l-lactic acid UV method; Boehringer Mannheim GmbH, Germany). After culturing for 24 h at 37°C, lactic acid concentrations in the CFCSs were as follows: L. rhamnosus GR1, 46 ± 6 mM; L. rhamnosus GG, 63 ± 8 mM; L. casei Shirota YT9029, 64 ± 12 mM; L. casei DN-114 001, 60 ± 20 mM; L. johnsonii La1, 61 ± 16 mM, and L. sakei CWBI 030202, 43 ± 2 mM.
Determination of the killing activity.
The serovar Typhimurium SL1344 culture was centrifuged at 5,500 × g for 5 min at 4°C. The culture medium was discarded, and the bacteria were washed once with PBS and resuspended in LB or Dulbecco's modified Eagle's minimum essential medium (DMEM; Invitrogen), as indicated. The bacteria were counted, and a volume containing 2 × 108 CFU/ml was used to determine the killing activity. A colony count assay was performed by incubating 500 μl of serovar Typhimurium SL1344 at 2 × 108 CFU/ml in LB or DMEM with 500 μl of CFCS, MRS broth, or MRS-LA at 37°C. At predetermined intervals, aliquots were removed, serially diluted, and plated on tryptic soy agar (Invitrogen) to determine the bacterial colony count.
Inhibition of growth.
Bacterial cultures were centrifuged at 5,500 × g for 5 min at 4°C, washed once with PBS, and suspended in LB or DMEM. A volume of 100 μl containing 2 × 105 CFU/ml of serovar Typhimurium SL1344 in LB or DMEM was mixed with 100 μl of Lactobacillus CFCS, MRS-HCl, or MRS-LA for 0 to 18 h at 37°C (pH of incubation medium, 4.5 ± 0.2). At predetermined times, the optical density at 612 nm was automatically determined using an automated GENios microtiter instrument (Tecan France S.A., Trappes, France).
Statistics.
Data are expressed as the means ± standard deviations of at least three separate duplicate experiments. The statistical significance was assessed by Student's t test. Differences were considered significant at a P value of <0.01.
RESULTS
Killing activity against S. enterica serovar Typhimurium SL1344.
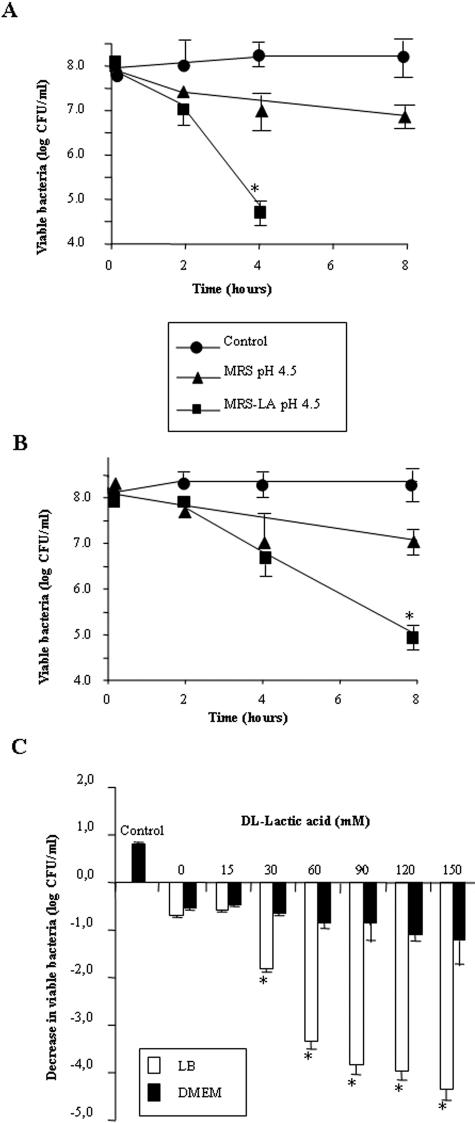
We conducted an experiment in order to distinguish between the killing activity attributable to the presence of lactic acid and that attributable to non-lactic acid molecule(s) in the CFCSs. As shown in Fig. 1A, a decrease of 3.5 logs in viable serovar Typhimurium SL1344 was observed when the killing activity of MRS-LA (60 mM d,l-lactic acid; pH of the incubation medium, 4.5 ± 0.2) was measured in the presence of LB. As shown in Fig. 1B, when DMEM was added to the incubation medium instead of LB (pH of the incubation medium, 4.5 ± 0.2), the data indicate that the killing effect of MRS-LA was delayed. Indeed, a reduced killing activity was observed after 4 h of contact (a decrease of 1 log in viable serovar Typhimurium SL1344 [Fig. 1B] compared to MRS-LA in the presence of LB [Fig. 1A]). However, a decrease of 3.0 logs in viable serovar Typhimurium SL1344 was observed for MRS-LA after 8 h of contact in the presence of DMEM (Fig. 1B), which was comparable to the activity of MRS-LA found in the presence of LB after 4 h of contact (Fig. 1A). It was noteworthy that MRS-HCl alone shows a low level of killing activity against serovar Typhimurium SL1344, with a decrease of 1.0 log after 4 h and 8 h of contact in the presence of both LB (Fig. 1A) and DMEM (Fig. 1B) (pH of the incubation medium, 4.4 ± 0.3).
FIG. 1.
Effect of DMEM on the killing effect of lactic acid against S. enterica serovar Typhimurium strain SL1344. (A) Killing effect in the presence of LB. (B) Killing effect in the presence of DMEM. (C) Concentration-dependent killing effect of lactic acid in the presence of LB or DMEM. The killing activity of d,l-lactic acid in the presence of LB or DMEM is shown. Values significantly different from control values are indicated by an asterisk (P < 0.01).
We next examined the inhibitory effect of increasing concentrations of lactic acid after 4 h of contact (concentrations ranging from 15 to 150 mM) (Fig. 1C). d,l-Lactic acid produced a dose-dependent killing activity in the presence of LB (pH of the incubation medium, 4.4 ± 0.4). In the presence of DMEM (pH of the incubation medium, 4.5 ± 0.2), lactic acid showed a decreased dose-dependent killing effect.
The killing activity of the strains L. johnsonii La1, L. rhamnosus GG, L. casei Shirota YT9029, L. casei DN-114 001, L. rhamnosus GR1, and L. sakei strain CWBI 030202 was examined in the presence of DMEM (Fig. 2A). The CFCSs of the probiotic La1, GG, YT9029, DN-114 001, and GR1 strains promoted a dramatic decrease in the viability of serovar Typhimurium SL1344 after 4 h of contact. In contrast, the food strain Lactobacillus sakei CWBI 030202, which decreased the viability of serovar Typhimurium SL1344 by 3 logs in the presence of LB (not shown), displayed no killing activity in the presence of DMEM (Fig. 2A). This result suggests that the production of an antibacterial non-lactic acid molecule(s) by Lactobacillus strains is strain specific.
FIG. 2.
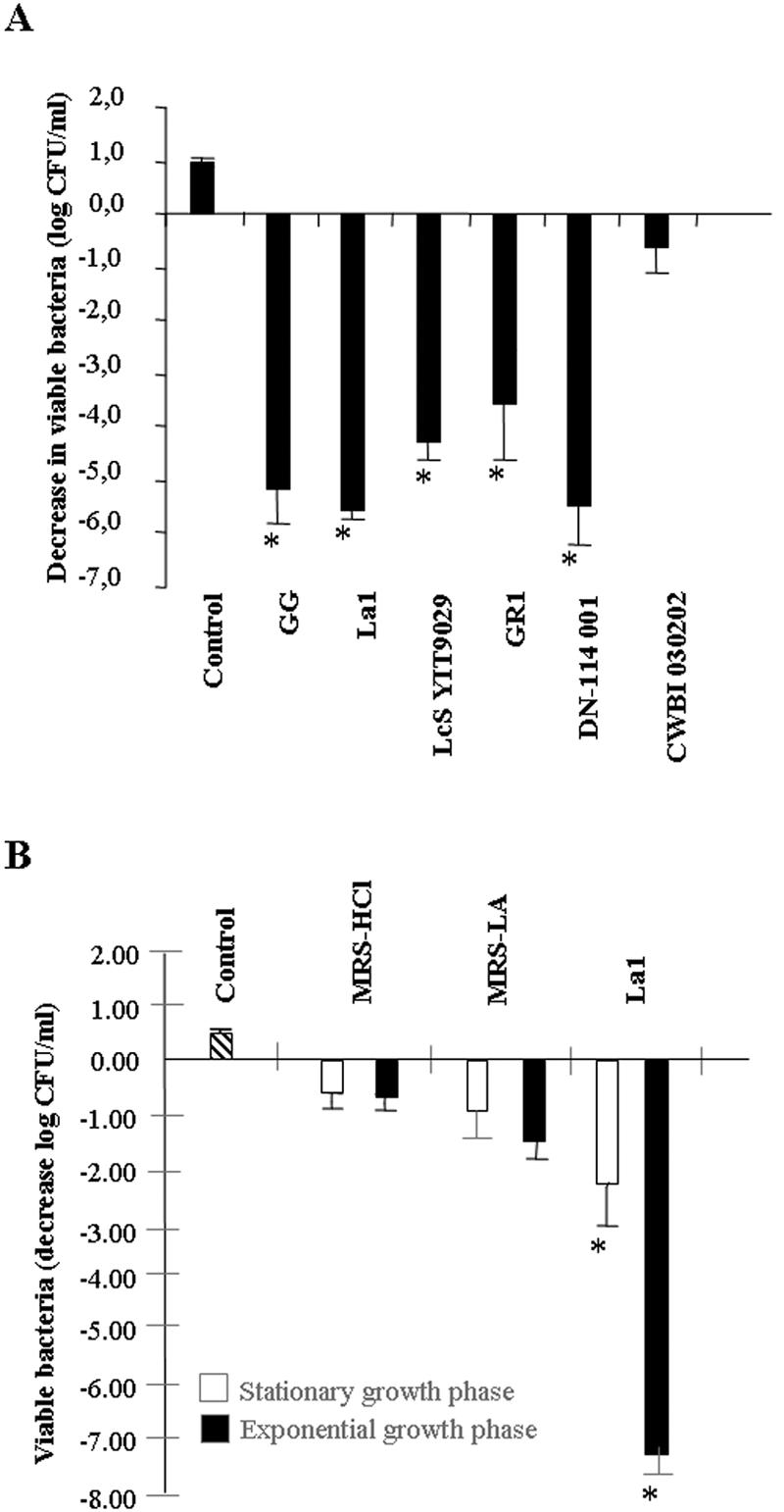
Killing activity of CFCSs of the probiotic Lactobacillus strains L. johnsonii La1, L. rhamnosus GG, L. casei Shirota YIT9029 (LcS YT9029), L. casei DN-114 001, L. rhamnosus GR1, and L. sakei CWBI 030202. (A) Killing activity of CFCSs in the presence of DMEM. (B) Effect of MRS-HCl, MRS-LA, and L. johnsonii La1 CFCS on the viability of Salmonella enterica serovar Typhimurium strain SL 1344 at the stationary or exponential growth phase. The killing effect was measured in the presence of DMEM. Values significantly different from control values are indicated by an asterisk (P < 0.01).
As can be seen in Fig. 2B, the La1 strain was more active against serovar Typhimurium SL1344 during the exponential phase of growth than during the stationary phase of growth. In contrast, MRS-HCl and MRS-LA both displayed similar activities against serovar Typhimurium SL1344 at all stages of growth.
Characteristics of killing activity.
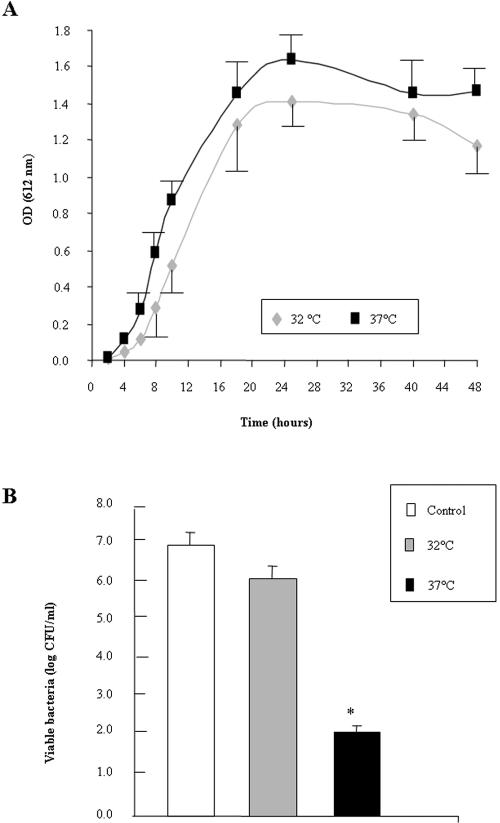
We investigated whether the temperature at which the probiotic Lactobacillus strains had been grown had any influence on the production of the non-lactic acid molecule(s) responsible for the killing activity. We used L. rhamnosus GG as the test strain. As shown in Fig. 3A, no significant difference was observed at the time points on the curves for the growth of GG bacteria at temperatures of 32°C and 37°C. A highly significant decrease in the serovar Typhimurium killing activity was observed when the GG bacteria were grown at 32°C compared to a growth temperature of 37°C (Fig. 3B), whereas the concentrations of lactic acid were similar (at 32°C, 59 ± 6 mM; at 37°C, 63 ± 8 mM).
FIG. 3.
Effect of growth temperature on the killing effect of L. rhamnosus GG CFCS. (A) Strain GG grown at 32°C and 37°C. (B) Killing effect in the presence of DMEM of CFCSs from strain GG obtained after the growth of bacteria at 32°C or 37°C. Values significantly different from control values are indicated by an asterisk (P < 0.01). OD, optical density.
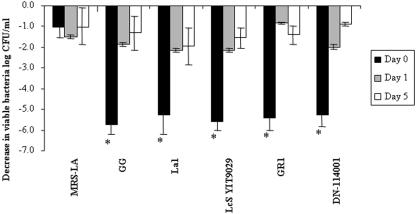
We found that the killing activities of the CFCSs obtained after 24 h of culture of the L. johnsonii La1, L. casei Shirota YT9029, L. casei DN-114 001, and L. rhamnosus GR1 strains rapidly decreased as a function of the number of days in storage at 4°C (Fig. 4). After 1 day of storage, CFCSs of the GG, L. casei Shirota YIT92029, La1, and DN-114001 strains displayed the same residual killing activity, with a decrease of 2 logs in Salmonella viability. In contrast, the killing activity of GR1 CFCS disappeared after 1 day of storage at 4°C, since the residual activity was indistinguishable from the activity of MRS-LA.
FIG. 4.
Development of the killing activity of the probiotic Lactobacillus strains L. johnsonii La1, L. rhamnosus GG, L. casei Shirota YIT9029 (LcS YT9029), L. casei DN-114 001, L. rhamnosus GR1, and L. sakei CWBI 030202 during storage at 4°C. CFCSs of probiotic Lactobacillus strains were obtained from a 24-h culture and kept at 4°C for 5 days. Killing activity against serovar Typhimurium SL1344 after 4 h of contact in the presence of DMEM was determined at day 0, day 1, and day 5 of storage. Values that were significantly different from values of controls are indicated by an asterisk (P < 0.01).
Inhibition of serovar Typhimurium SL1344 growth.
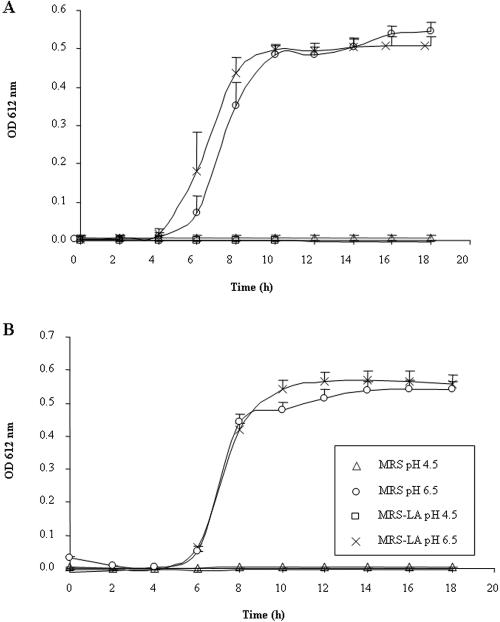
We conducted experiments to investigate the effect of pH and of lactic acid on the growth of serovar Typhimurium SL1344. As shown in Fig. 5, under control conditions, the growth of serovar Typhimurium SL1344 began after 4 h, developed rapidly, stabilized after 10 h, and did not develop any further after that. When serovar Typhimurium SL1344 was cultured in the presence of MRS-HCl at pH 4.5, no bacterial growth was observed in the presence of either LB (Fig. 5A) or DMEM (Fig. 5B). In the presence of LB (Fig. 5A) or DMEM (Fig. 5B), MRS-LA at pH 4.5 inhibited the growth of serovar Typhimurium SL1344 to the same extent. When MRS-HCl and MRS-LA were adjusted to pH 6.5, serovar Typhimurium SL1344 growth occurred. Similarly, all the CFCSs of the L. johnsonii La1, L. casei Shirota YT9029, L. casei DN-114 001, L. rhamnosus GR1, and L. sakei CWBI 030202 strains inhibited serovar Typhimurium SL1344 growth at pH 4.5 but did not display any inhibitory activity at pH 6.5 (not shown).
FIG. 5.
The inhibition of the growth of S. enterica serovar Typhimurium strain SL1344 is pH dependent. Serovar Typhimurium SL1344 (5 × 105 CFU/ml) was exposed to MRS-HCl (pH 4.5 or pH 6.5), or MRS-LA (60 mM, pH 4.5) in the presence of LB (A) or DMEM (B) for 0 to 18 h at 37°C. There was a significant difference (P < 0.01) between MRS-HCl (pH 6.5) or MRS-LA (60 mM, pH 6.5) and MRS-HCl (pH 4.5) or MRS-lactic acid (60 mM, pH 4.5), respectively, in the presence of LB (A) or DMEM (B). OD, optical density.
DISCUSSION
It has been previously reported that the antibacterial activity of probiotic lactic acid strains generally results from the production of H2O2, acids, strain-specific metabolites, bacteriocins, or non-lactic acid molecule(s) (24). Killing activity of probiotic Lactobacillus and Bifidobacterium strains has been previously investigated using an in vitro method in which activity was measured in the presence of LB or PBS (2, 5, 15). By adding DMEM to the incubating medium, we provide evidence that only the killing activity of non-lactic acid molecule(s) should be measured after 4 h of contact, since DMEM delays the killing activity of lactic acid. But, it remains to be determined whether DMEM delays the killing activity of lactic acid. In addition, we observed that the production of a non-lactic acid molecule(s) that supports killing activity is dependent on the temperature of growth. Finally, on the basis of the observation that a significant part of the killing activity had disappeared after 1 day of storage at 4°C, we believe that both an unstable and a stable molecular species responsible for the killing activity against Salmonella were produced in the CFCS by the probiotic Lactobacillus strains.
It has been previously reported that the probiotic strains L. johnsonii La1 (2, 18, 27), L. rhamnosus GG (11-14, 16, 26, 27), L. casei Shirota YT9029 (12, 13, 20, 25, 27), and L. rhamnosus GR1 (4, 17, 22) exert antagonistic activity against gram-negative pathogens. We observed that the La1, GG, YT9029, GR1, and DN-114001 strains acted mainly by secreting a non-lactic acid molecule(s) into the CFCS. Previous reports have indicated that some of the non-lactic acid antibacterial molecules produced by probiotic Lactobacillus strains and which are active against gram-negative pathogens display nonbacteriocin characteristics, although most of these molecules remain to be identified (15). For the strain L. rhamnosus GR1, a bactericidal substance that is neither lactic acid nor hydrogen peroxide has been observed. The substance has a molecular mass greater than 12 to 14 kDa, is heat labile, is not precipitated by up to 80% ammonium sulfate, and is extractable in chloroform (17). The CFCSs of the strains Lactobacillus acidophilus LB and L. johnsonii La1 contain a nonbacteriocin, non-lactic acid antibacterial molecule(s) that is heat stable and insensitive to proteases (2, 5). For the strain L. rhamnosus GG, a secreted, low-molecular-mass, heat-stable, inhibitory substance has been described that is distinct from lactic and acetic acids (26). Lehto and Salminen (14) have proposed that L. rhamnosus GG has a pH-dependent antibacterial effect against Salmonella, since they observed that the effect of GG culture was abolished when the pH was adjusted to 7. The substances with antagonist activities against Escherichia coli strain O157:H7 by the strains L. rhamnosus DR20 and L. acidophilus HN017 were partially inactivated by treatment with lactate dehydrogenase, suggesting the presence of a non-lactic acid molecule(s) (10). It is interesting to note that the results reported here show that killing activity against Salmonella develops to a greater extent during the exponential growth phase than during stationary growth. This is a characteristic previously reported for defensins which are known to contribute to the host's mucosal defense in the small intestine (9). Indeed, metabolically active bacteria are more sensitive to defensins than inactive bacteria.
It has previously been reported that Lactobacillus strains inhibit the growth of gram-negative pathogenic bacteria (28). This growth-inhibiting activity has generally been attributed to the fact that Lactobacillus spp. lower the pH and/or produce lactic acid. For example, strains of L. acidophilus, L. casei subsp. rhamnosus, and Lactobacillus bulgaricus inhibited the growth of clinical isolates of H. pylori (3, 19) and L. casei subsp. rhamnosus strain Lcr35 reduced the growth of enteropathogenic Escherichia coli, enterotoxigenic E. coli, and Klebsiella pneumoniae (8). The data reported here show that Lactobacillus strains induce complete inhibition of the growth of serovar Typhimurium SL1344 that results mainly from the effect of an acid pH. Indeed, when we examined the inhibition of the growth of serovar Typhimurium SL1344 by lactic acid and by the probiotic strains L. johnsonii La1, L. rhamnosus GG, L. casei Shirota YT9029, L. casei DN-114 001, and L. rhamnosus GR1, we found that inhibition occurred when the pH of the incubation medium was acid and that no growth inhibition occurred when the pH of the incubation medium was neutral. Our findings are in agreement with those reported by Ogawa et al. (21) showing that L. casei Shirota YT9029 reduced the growth of Shiga-toxin-producing E. coli O157:H7 as a result of the production of lactic acid and a pH-lowering effect.
In conclusion, we provide evidence that the killing effect of probiotic Lactobacillus strains against S. enterica serovar Typhimurium results mainly from the strain-specific non-lactic acid molecule(s) present in their CFCSs. However, the antibacterial activity of probiotic lactic acid strains is known to be multifactorial (24). In particular, it has been demonstrated that lactic acid participates in the antibacterial activity of probiotic Lactobacillus strains. It is noteworthy that l-lactic acid displays a greater antibacterial activity than d-lactic acid or d,l-lactic acid. In consequence, the higher antibacterial activity of probiotic Lactobacillus strains that produced l-lactic acid could be related in part to the greater proportion of l-lactate in the CFCSs than in strains producing d,l-lactic acid. Moreover, it was noted that the mechanism of the antibacterial activity of probiotic Lactobacillus strains that leads to the killing of bacterial pathogens may be due to a synergistic action of lactic acid and the secreted non-lactic acid molecules. This synergism has been previously reported by Alakomi et al. (1), who showed that the lactic acid acts as a permeabilizer of the outer membrane of gram-negative pathogens, thus increasing their susceptibility to antimicrobial molecules by allowing these molecules to penetrate the bacteria.
Acknowledgments
D. Fayol-Messaoudi and C. N. Berger were supported by the QLK1-2001-01179 PROPATH Program (Commission of the European Communities).
Part of this study was carried out with financial support from the Commission of the European Communities, specifically by the RTD program “Quality of Life and Management of Living Resources,” QLK1-2001-01179 “PROPATH”—Molecular analysis and mechanistic elucidation of the functionality of probiotics and prebiotics in the inhibition of pathogenic microorganisms to combat gastrointestinal disorders and to improve human health. It does not necessarily reflect its views and in no way anticipates the Commission's future policy in this area.
REFERENCES
- 1.Alakomi, H. L., E. Skytta, M. Saarela, T. Mattila-Sandholm, K. Latva-Kala, and I. M. Helander. 2000. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66:2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernet-Camard, M. F., V. Lievin, D. Brassart, J. R. Neeser, A. L. Servin, and S. Hudault. 1997. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl. Environ. Microbiol. 63:2747-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia, S. J., N. Kochar, P. Abraham, N. G. Nair, and A. P. Mehta. 1989. Lactobacillus acidophilus inhibits growth of Campylobacter pylori in vitro. J. Clin. Microbiol. 27:2328-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, R. C., G. Reid, R. T. Irvin, A. W. Bruce, and J. W. Costerton. 1985. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect. Immun. 47:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coconnier, M. H., V. Lievin, M. F. Bernet-Camard, S. Hudault, and A. L. Servin. 1997. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob. Agents Chemother. 41:1046-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay, B. B., and S. Falkow. 1990. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162:1096-1106. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations/World Health Organization. 2001. FAO/WHO expert consultation evaluation of health and nutritional properties of powder milk with live lactic acid bacteria, Cordoba, Argentina—1 to 4 October 2001. http://www.fao.org/es/esn/food/foodandfood_probiocons_en.stm.
- 8.Forestier, C., C. De Champs, C. Vatoux, and B. Joly. 2001. Probiotic activities of Lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res. Microbiol. 152:167-173. [DOI] [PubMed] [Google Scholar]
- 9.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 10.Gopal, P. K., J. Prasad, J. Smart, and H. S. Gill. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216. [DOI] [PubMed] [Google Scholar]
- 11.Hudault, S., V. Lievin, M. F. Bernet-Camard, and A. L. Servin. 1997. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol. 63:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, Y. K., and K. Y. Puong. 2002. Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Br. J. Nutr. 88(Suppl. 1):S101-S108. [DOI] [PubMed] [Google Scholar]
- 13.Lee, Y. K., K. Y. Puong, A. C. Ouwehand, and S. Salminen. 2003. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52:925-930. [DOI] [PubMed] [Google Scholar]
- 14.Lehto, E. M., and S. J. Salminen. 1997. Inhibition of Salmonella typhimurium adhesion to Caco-2 cell cultures by Lactobacillus strain GG spent culture supernate: only a pH effect? FEMS Immunol. Med. Microbiol. 18:125-132. [DOI] [PubMed] [Google Scholar]
- 15.Lievin, V., I. Peiffer, S. Hudault, F. Rochat, D. Brassart, J. R. Neeser, and A. L. Servin. 2000. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 47:646-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack, D. R., S. Michail, S. Wei, L. McDougall, and M. A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941-G950. [DOI] [PubMed] [Google Scholar]
- 17.McGroarty, J. A., and G. Reid. 1988. Detection of a Lactobacillus substance that inhibits Escherichia coli. Can. J. Microbiol. 34:974-978. [DOI] [PubMed] [Google Scholar]
- 18.Michetti, P., G. Dorta, P. H. Wiesel, D. Brassart, E. Verdu, M. Herranz, C. Felley, N. Porta, M. Rouvet, A. L. Blum, and I. Corthesy-Theulaz. 1999. Effect of whey-based culture supernatant of Lactobacillus acidophilus (johnsonii) La1 on Helicobacter pylori infection in humans. Digestion 60:203-209. [DOI] [PubMed] [Google Scholar]
- 19.Midolo, P. D., J. R. Lambert, R. Hull, F. Luo, and M. L. Grayson. 1995. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 79:475-479. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa, M., K. Shimizu, K. Nomoto, M. Takahashi, M. Watanuki, R. Tanaka, T. Tanaka, T. Hamabata, S. Yamasaki, and Y. Takeda. 2001. Protective effect of Lactobacillus casei strain Shirota on Shiga toxin-producing Escherichia coli O157:H7 infection in infant rabbits. Infect. Immun. 69:1101-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa, M., K. Shimizu, K. Nomoto, R. Tanaka, T. Hamabata, S. Yamasaki, T. Takeda, and Y. Takeda. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic Lactobacillus strains due to production of lactic acid. Int. J. Food Microbiol. 68:135-140. [DOI] [PubMed] [Google Scholar]
- 22.Reid, G., R. L. Cook, and A. W. Bruce. 1987. Examination of strains of lactobacilli for properties that may influence bacterial interference in the urinary tract. J. Urol. 138:330-335. [DOI] [PubMed] [Google Scholar]
- 23.Reid, G., J. Jass, M. T. Sebulsky, and J. K. McCormick. 2003. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16:658-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 25.Sgouras, D., P. Maragkoudakis, K. Petraki, B. Martinez-Gonzalez, E. Eriotou, S. Michopoulos, G. Kalantzopoulos, E. Tsakalidou, and A. Mentis. 2004. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl. Environ. Microbiol. 70:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva, M., N. Jacobus, C. Deneke, and S. L. Gorbach. 1987. Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother. 31:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 1999. The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol. Med. Microbiol. 26:137-142. [DOI] [PubMed] [Google Scholar]
- 28.Vandenbergh, P. A. 1993. Lactic acid bacteria, their metabolic products and interference with microbial growth. FEMS Microbiol. Rev. 12:221-238. [Google Scholar]