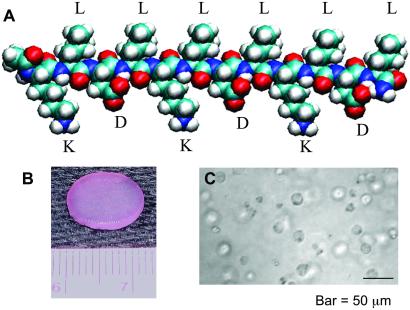
Figure 1.
(A) Molecular model of a single KLD-12 self-assembling peptide. The alternating hydrophobic and hydrophilic residues on the backbone promote β-sheet formation. The positively charged lysines (K) and negatively charged aspartic acids (D) are on the lower side of the β-sheet, and the hydrophobic leucines (L) are on the upper side. This molecular structure facilitates self-assembly through intermolecular interactions. (B) A 12-mm chondrocyte-seeded peptide hydrogel plug, punched from 1.6-mm-thick slabs. (C) Light microscope image of chondrocytes encapsulated in peptide hydrogel.