Fig. 5. Binding of caldesmon to unmodified, modified, and cleaved actin.
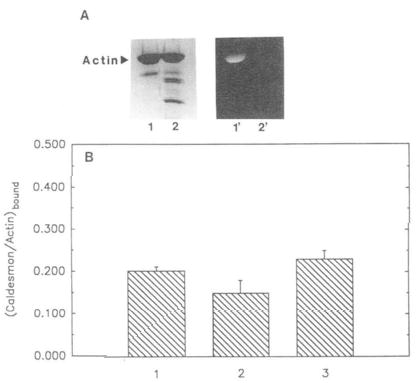
1,5-IAEDANS-labeled actin (at Cys-374) and tryptically cleaved F-actin (to remove the COOH-terminal residues) were prepared as described under “Materials and Methods.” A, lanes 1 and 2, Coomassie stained, 1,5-IAEDANS modified, uncleaved and cleaved actin, respectively. Lanes 1′ and 2′ correspond to the same bands viewed under UV illumination. B, comparison of caldesmon binding to unmodified, modified, and cleaved actin. Unmodified, modified, and cleaved actin (4.0 μm each) were allowed to independently incubate with caldesmon (1.5 μm) Pelleted samples from co-sedimentation experiments (see “Materials and Methods’) were run on SDS gels, Coomassie stained, and analyzed by densitometer. Molar ratios of caldesmon bound to actin are represented in the form of a bar graph. 1, caldesmon bound to unmodified actin (0.20 ± 0.01); 2, caldesmon bound to 1,5-IAEDANS modified (uncleaved) actin (0.15 ± 0.03); 3, caldesmon bound to 1,5-IAEDANS modified (cleaved) actin (0.23 ± 0.02). Standard deviation (n = 5) is indicated by error bars above each bar column.
