Abstract
We examine the IFN-α/β-independent activation of cellular transcription that constitutes an early antiviral response of cells against influenza A and B viruses, which cause widespread epidemics in humans. We show that influenza B virus induces the synthesis in human cells of several mature mRNAs encoded by genes containing an IFN-α/β-stimulated response element (ISRE). Consequently, the IFN regulatory factor-3 transcription factor, which is required for the transcription of ISRE-controlled genes, is activated after influenza B virus infection. The production of these cellular mRNAs, some of which encode antiviral proteins, is independent of not only IFN-α/β, but also viral protein synthesis. These mature cellular antiviral mRNAs are not produced after infection with influenza A virus, but IFN regulatory factor-3 is activated and the transcription of the ISRE-controlled p56 gene is induced. Consequently, like other newly synthesized cellular premRNAs in influenza A virus-infected cells, the posttranscriptional processing of premRNAs encoded by ISRE-controlled genes is inhibited. Previous work has established that such posttranscriptional inhibition is mediated by the viral NS1A protein. This unique, global countermeasure against the early, IFN-α/β-independent antiviral response of cells may be an important factor in the pathogenicity of influenza A virus infection.
Mammalian cells respond to virus infection by activating transcription factors that can enable the cells to survive infection. One set of transcription factors, IFN regulatory factor-3 (IRF-3) and IRF-7, is directly activated after infection with both DNA and RNA viruses (1–13). This activation is not mediated by IFN-α/β and does not require viral protein synthesis. Activated IRF-3 and IRF-7 combine with the transcriptional coactivators p300 and CREB-binding protein (CBP) to form a transcription complex. This complex (virus-activated factor) binds to a subset of the IFNα/β-stimulated response elements (ISREs) present in the promoters of particular cellular genes, thereby inducing their transcription (4–6, 11, 13, 14). These cellular transcripts are processed to form mature mRNAs (3, 7, 9, 12). Some of the proteins encoded by these genes have antiviral activities that can protect initially infected cells against virus replication (7, 9, 12), indicating this early virus-induced, IFN-α/β-independent activation of cellular transcription constitutes the initial antiviral response of cells. Viruses can be presumed to mount countermeasures against such an early antiviral response to allow the virus to replicate (9).
In addition, infection of mammalian cells by many viruses activates the transcription factors that bind to the enhancer elements of the IFN-α and IFN-β genes, thereby inducing their transcription (15). The complex of transcription factors required for the induction of IFN-β transcription contains two of the factors present in the virus-activated factors, IRF-3 and IRF-7, and additional factors (5, 16–18). The IFN-α/β protein molecules synthesized in initially infected cells are secreted and bind to cell-surface receptors to establish an antiviral state by the activation of transcription factors (15). Activation results in the formation of a protein complex, called ISGF-3 (19), which induces the transcription of all ISRE-controlled cellular genes (5, 14). Secreted IFN-α/β may not provide much protection to initially infected cells that produce IFN-α/β because these cells already have an established virus infection. Rather, it is likely that the primary role of IFN-α/β is to protect neighboring uninfected cells, thereby inhibiting virus spread.
An important unresolved issue is whether the early, IFN-α/β-independent activation of transcription of ISRE-controlled cellular genes is an event that is common to infections by all mammalian viruses. This issue has arisen in part because it has been reported that infection of mammalian cells with influenza A virus, a segmented negative-strand RNA virus, does not activate IRF-3 (20), which is required for transcription of these cellular antiviral genes (4–6, 11, 13, 14), and for transcription of the IFN-α and IFN-β genes (5, 17). In contrast, in mammalian cells infected with the closely related influenza B virus, the transcription of at least one ISRE-controlled cellular gene (encoding the ISG15 protein) has been shown to be activated (21).
Influenza A and influenza B viruses, two of the four influenza virus genera, cause widespread epidemics in humans (22, 23). Influenza A viruses, which have been isolated from a wide variety of avian and mammalian species, are responsible for the human pandemics that have caused high mortality rates, and the highly pathogenic virus that was transmitted from chickens to humans in Hong Kong in 1997 is an influenza A virus (23). Influenza B virus seems to infect only humans (23), although influenza B virus has recently been isolated from seals (24). Both influenza A and B viruses contain eight genomic RNA segments, and most of the proteins encoded by the corresponding genomic RNA segments serve similar functions (22). However, the sizes of both the genomic RNA segments and their encoded proteins differ between influenza A and B viruses, and differences occur in the mechanisms of expression of two viral genes, the M (matrix) and NA (neuraminidase) genes (22).
In addition, the biological activities of the nonstructural protein 1 (NS1 protein) encoded by influenza A virus differ markedly from the activities of the NS1 protein encoded by influenza B virus (21, 25–27). The NS1 protein of influenza A virus (NS1A protein), but not the NS1 protein of influenza B virus (NS1B protein), binds and inhibits the function of two cellular proteins that are required for the 3′ end processing of cellular premRNAs: the 30-kDa subunit of the cleavage and polyadenylation specificity factor and poly(A)-binding protein II (26–28). As a result of the inhibition of 3′ end processing, cellular mRNAs are not exported from the nucleus after influenza A virus infection (27, 29), and cellular premRNAs accumulate in the nucleus of infected cells (27). By using influenza A viruses that encode mutant NS1A proteins, it was verified that the inhibition of these cellular posttranscriptional events in infected cells is mediated by the NS1A protein (27, 29). These results explain the earlier observation that the nuclear export of newly synthesized cellular mRNAs is blocked in influenza A virus-infected cells (30).
In the present study we demonstrate that influenza A virus as well as influenza B virus induces the IFN-α/β-independent activation of the transcription of ISRE-controlled cellular genes in human cells, thereby establishing that both of these human influenza viruses activate IRF-3. Further, we show that influenza A virus is unique in that induction of this cellular transcription does not result in the production of mature cellular antiviral mRNAs. Hence, these results indicate that the posttranscriptional processing of newly synthesized premRNAs encoded by ISRE-controlled genes is inhibited, as are other newly synthesized cellular premRNAs in influenza A virus-infected cells (26–30). As discussed above, this inhibition has been shown to be mediated by the viral NS1A protein (27, 29). This global, posttranscriptional countermeasure against the early, IFN-α/β-independent antiviral response of cells may be an important factor in the pathogenicity of influenza A virus infection.
Materials and Methods
Viruses.
Influenza A/Udorn/72 and B/Lee/40 virus stocks were grown in 11-day fertilized eggs, and virus titers were determined by plaque assays in Madin–Darby canine kidney (MDCK) cells. Where indicated, these influenza viruses were irradiated with 360 mJ of UV light by using a Stratalinker 2400.
Northern Analysis and Western Immunoblotting.
The steady-state levels of ISG15, p56, and 2′-5′ oligo(A) synthetase (2–5 OAS) mRNAs in mock-infected and influenza virus-infected cells were determined by Northern analysis of total cellular RNAs. To prepare the labeled probes for these mRNAs, the cDNA sequences in the ISG15-pGEM-1 (21), p56-pBKS(II) (8), and 2–5 OAS-pGEM4 (31) plasmids were each amplified by PCR, followed by a random primer-labeling reaction by using the Klenow fragment of DNA polymerase I. The amounts of the ISG15, p56, and 2–5 OAS proteins were determined by Western immunoblotting performed as described (21). The immunoblots were probed with ISG15 antiserum (32), p56 antiserum (8), or 2–5 OAS antiserum (33), and antibody complexes were identified by using ECL Western blotting reagents (Amersham Pharmacia).
Assays for the Activation of IRF-3.
Mock-infected and influenza virus-infected cells were suspended in a buffer containing 50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, and a mixture of protease and phosphatase inhibitors. After vortexing, the mixture was maintained at 4°C for 10 min and centrifuged at 10,000 × g for 5 min. This procedure was sufficient to extract IRF-3-containing transcription complexes from the nuclei of GRE cells, whereas a higher salt concentration (400 mM NaCl) was needed to extract these complexes from the nuclei of A549 cells. For the IRF-3 dimerization assay, an aliquot of the cell extract (30 μg of protein) was treated with 0.5–1% deoxycholate and, where necessary, the final NaCl concentration was reduced to 75 mM. The mixture was subjected to electrophoresis on a 7.5% native gel to resolve the monomer and dimer forms of IRF-3, as described by Iwamura et al. (10). IRF-3 monomers and dimers were detected by Western immunoblotting by using the SL-12 monoclonal antibody (34). The second assay for IRF-3 activation measured the association of IRF-3 with CBP by coimmunoprecipitation. The cell extract was immunoprecipitated with the SL-12 antibody, followed by electrophoresis on a 6% denaturing SDS-polyacrylamide gel and immunoblotting with CBP antibody (Santa Cruz Biotechnology).
Preparation of Nuclei and in Vitro Runoff Assays.
Procedures were as described by Ausubel et al. (35), with some modifications. A549 cells (5 × 107) were mock-infected, or were infected with either influenza A/Udorn/72 or influenza B/Lee/40 virus at a multiplicity of infection of 7. Four hours later cells were removed from the culture dishes by trypsinization, and washed sequentially with cold PBS and RSB (10 mM Tris⋅HCl, pH 7.5/10 mM NaCl/5 mM MgCl2). The cells were then resuspended in 4 ml of RSB and allowed to swell for 10 min on ice. After Dounce homogenization, the nuclei were collected by centrifugation at 500 × g for 5 min. The nuclei were resuspended in an equal volume of 2× transcription buffer (20 mM Tris⋅HCl, pH 7.5/50 mM DTT/180 mM KCl/10 mM MgCl2). Transcription reactions contained: a 200-μl aliquot of this nuclear preparation; ATP, CTP, and GTP (each at 1 mM); and 250 μCi (1 Ci = 37 GBq) of (α-32P)UTP (3,000 Ci/mmol). Reactions were incubated at 30°C for 30 min, after which RNA was extracted with Trizol reagent (GIBCO) and precipitated with ethanol. The RNA was dissolved in a small volume (30 μl) of water, and after the addition of 400 μl of Ekono hybridization solution (Research Products International), was hybridized to p56 cDNA affixed to a nylon membrane in a dot blot apparatus. The p56 cDNA was obtained by PCR amplification of the p56 cDNA sequence in the p56-pBKS(II) plasmid, and was UV light-crosslinked to the nylon membrane. The filters were first incubated with 1 ml of Ekono solution for 2 h at 65°C, and were hybridized with the RNA products of the runoff reaction for 40 h at 65°C. After extensive washing, the filters were exposed to x-ray film.
Results
Influenza B Virus, but Not Influenza A Virus, Induces an IFNα/β-Independent Production of Mature mRNAs Encoded by ISREControlled Cellular Genes.
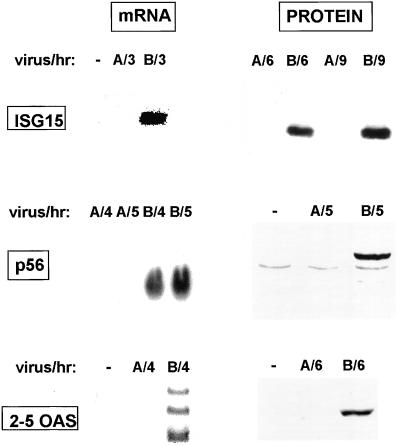
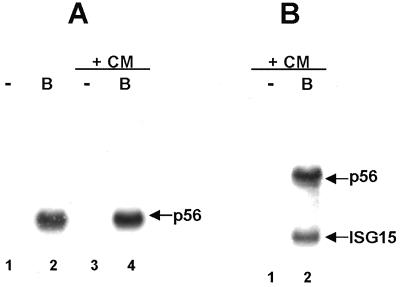
As the first approach for determining whether the transcription of ISRE-controlled genes is activated in cells infected by influenza A virus or influenza B virus, we used Northern analysis to measure the production of three mRNAs in A549 human lung cells before and after virus infection: the ISG15, p56, and 2–5 OAS mRNAs, which are encoded by ISRE-controlled genes whose transcription is activated by infection with other viruses (4–6, 11–14). These three mRNAs are not produced in mock-infected cells or in cells after infection with the human influenza A/Udorn/72 virus, but are efficiently produced in cells infected with the human influenza B/Lee/40 virus (Fig. 1). Consequently, influenza B virus activates the transcription of the ISG15, p56, and 2–5 OAS genes, and hence activates the factors, including IRF-3, that are required for this transcription. The proteins encoded by these mRNAs are synthesized in influenza B virus-infected cells (Fig. 1), indicating that the mRNAs that are detected by Northern analysis are functional. The synthesis of these cellular proteins can be considered to constitute an antiviral response by the cells, because at least one of these proteins, 2–5 OAS, has been shown to inhibit the replication of several viruses (36). Synthesis of the ISG15, p56, and 2–5 OAS proteins does not occur in cells infected by influenza A virus, as expected because of the absence of the mRNAs encoding these proteins (Fig. 1). The activation of transcription of ISRE-controlled genes induced by other viruses is not mediated by IFN-α/β and does not require viral protein synthesis (1–5, 7, 11–14, 37). To determine whether this lack of mediation by IFN-α/β is the case for influenza B virus infection, we used human GRE cells in which portions of the IFN-α and IFN-β genes are deleted (37). Efficient production of p56 and ISG15 mRNAs occur in GRE cells infected with influenza B virus both in the absence and presence of the protein synthesis inhibitor cycloheximide (CM) (Fig. 2 A and B). Thus, activation of the transcription of ISRE-controlled genes by influenza B virus is not mediated by IFN-α/β and does not require the synthesis of virus-encoded proteins.
Figure 1.
Influenza B virus, but not influenza A virus, induces the production of mature, functional mRNAs encoded by ISRE-controlled cellular genes. A549 cells were either mock-infected (−), infected with influenza A virus (designated A), or infected with influenza B virus (designated B). Infected cells were collected at the indicated times after infection. ISG15, p56, and 2–5 OAS mRNAs were detected by Northern blot analysis, and the encoded proteins were detected by Western immunoblotting.
Figure 2.
Activation of the transcription of ISRE-controlled genes by influenza B virus is not mediated by IFN-α/β and does not require the synthesis of viral proteins. GRE cells were either mock-infected (−) or were infected with influenza B virus (B) in the absence or presence of 100 μg/ml of CM for 4 h. Northern blot analysis was used to detect either only p56 mRNA (A) or both p56 and ISG15 mRNAs (B).
Influenza A Virus, Like Influenza B Virus, Induces an IFN-α/β-Independent Activation of IRF-3 and of the Transcription of ISRE-Controlled Cellular Genes.
Several explanations can be proposed for the lack of production of mature ISG15, p56, and 2–5 OAS mRNAs in cells infected by influenza A virus. One possibility is that IRF-3 is not activated after infection by influenza A virus (20). If this possibility were the case, transcription of the ISG15, p56, and 2–5 OAS genes would not occur. We measured the activation of IRF-3 by using two documented assays (5, 10, 13).
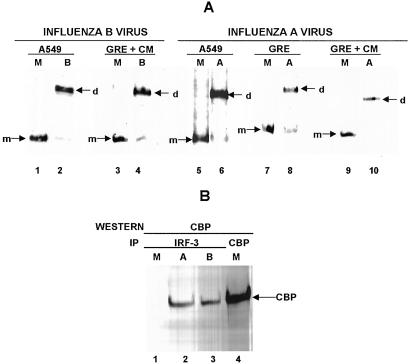
One assay measures the formation of the homodimer of IRF-3 that results from the phosphorylation-dependent activation of IRF-3 (10). Treatment of cell extracts with deoxycholate selectively removes p300 and CBP from IRF-3 dimers (5, 10), and nondenaturing electrophoresis is then used to separate activated IRF-3 dimers from nonactivated monomers (10). The detection of IRF-3 dimers depends on their efficient extraction from the nucleus, which varies to a certain extent between different experiments. This assay detects the activation of IRF-3 that, as shown above, occurs in cells infected by influenza B virus: all of the IRF-3 molecules from mock-infected A549 cells migrate in the position of the monomer (Fig. 3A, lane 1), whereas the majority of IRF-3 molecules in influenza B virus-infected A549 cells at 4 h after infection migrate in the position of the dimer (lane 2). Similar efficient IRF-3 dimer formation also occurs in GRE cells (which do not produce IFN-α/β) treated with CM at the time of influenza B virus addition (lanes 3 and 4). Thus, this assay detects the activation of IRF-3 that occurs in influenza B virus-infected cells, and verifies that this activation is independent of both IFN-α/β and the synthesis of viral proteins.
Figure 3.
Activation of IRF-3 by either influenza A virus or influenza B virus is not mediated by IFN-α/β and does not require the synthesis of virus-encoded proteins. (A) IRF-3 dimerization assay. A549 cells were either mock-infected (M, lanes 1 and 5), or infected for 4 h with either influenza B virus (B, lane 2) or influenza A virus (A, lane 6). GRE cells were either mock-infected (M, lane 7), or infected for 4 h with influenza A virus (A, lane 8). GRE cells in the presence of CM were either mock-infected (M, lanes 3 and 9), or infected for 4 h with either influenza B virus (B, lane 4) or influenza A virus (A, lane 10). An aliquot of the cell extracts was treated with deoxycholate and subjected to electrophoresis on a 7.5% native gel. The monomer (m) and dimer (d) forms of IRF-3 were identified by immunoblotting with the SL-12 monoclonal antibody. (B) Association of CBP with IRF-3 after influenza A or B virus infection. A549 cells were either mock-infected (M, lane 1), or infected for 4 h with either influenza A virus (lane 2) or influenza B virus (B, lane 3). Cell extracts were immunoprecipitated with the SL-12 antibody (anti-IRF-3), and immunoprecipitates were analyzed by Western blots with CBP antibody. Lane 4, cell extract from mock-infected cells was immunoprecipitated with CBP antibody to provide a marker lane for CBP.
Similar results were obtained with influenza A virus. All of the IRF-3 molecules from mock-infected A549 and GRE cells migrate in the position of the monomer (Fig. 3A, lanes 5 and 7), whereas most IRF-3 molecules in influenza A virus-infected A549 and GRE cells at 4 h after infection migrate in the position of the dimer (lanes 6 and 8). Efficient IRF-3 dimer formation also occurs in GRE cells treated with CM at the time of influenza A virus addition (lanes 9 and 10). These results indicate that IRF-3 activation also occurs in cells infected by influenza A virus, and that this activation is not mediated by IFN-α/β and does not require the synthesis of influenza A virus-encoded proteins.
The second assay for IRF-3 activation measured the association of IRF-3 with CBP, based on the demonstration that the virus-activated factor formed after infection by other viruses contains IRF-3 complexed with CBP (5, 13). Extracts from mock-infected cells and from cells infected with either influenza A or influenza B virus were immunoprecipitated with an IRF-3-specific antibody, and the immunoprecipitates were analyzed by Western blotting by using CBP-specific antibody. As shown in Fig. 3B, IRF-3 is associated with CBP after infection by either influenza A or influenza B virus (lanes 2 and 3), but not after mock infection (lane 1). These results thus confirm that IRF-3 is activated after influenza A virus infection.
These results, which demonstrate that IRF-3 is activated in influenza A virus-infected cells, strongly suggest that the transcription of ISRE-controlled genes is also activated in cells infected by influenza A virus. To determine whether this prediction is the case, we measured the transcription of the ISRE-controlled p56 gene directly by using nuclear runoff transcription assays. Nuclei isolated from mock-infected A549 cells and from influenza A virus-infected cells at 4 h after infection were incubated with (α-32P)UTP and unlabeled CTP, GTP, and ATP to label nascent RNA transcripts, and the labeled RNA was hybridized to p56 cDNA immobilized on a nylon membrane. Because this p56 cDNA was obtained by PCR amplification, all of the DNA on the nylon membrane is p56-specific. As a control, we also measured the transcription of the p56 gene at 4 h after infection by influenza B virus. As shown in Fig. 4A, transcription of the p56 gene, which does not occur in mock-infected cells, is substantially activated at 4 h after influenza A virus infection, and after influenza B virus infection. These results demonstrate that infection by influenza A virus activates the transcription of a cellular ISRE-controlled gene, thereby establishing that the IRF-3 which is activated after influenza A virus infection functions in transcription. Consequently, we conclude that the absence of mature cellular mRNAs encoded by these cellular genes results from the inhibition of the posttranscriptional processing of the premRNAs transcribed from these genes.
Figure 4.
(A) Influenza A virus, and influenza B virus, activates the transcription of the ISRE-controlled p56 gene. A549 cells were either mock-infected, infected with influenza A virus for 4 h, or infected with influenza B virus for 4 h. Nuclei from these cells were incubated with (α-32P)UTP and unlabeled CTP, GTP, and ATP, and the labeled RNA was hybridized to p56-specific cDNA immobilized on a nylon membrane in a dot blot apparatus. Control experiments showed that labeled RNA did not bind to nylon membranes containing a plasmid DNA lacking p56 sequences. (B) At least one influenza A virus-encoded protein mediates the inhibition of the posttranscriptional processing of p56 and ISG15 premRNAs. GRE cells in the presence of CM were either mock-infected (M, lanes 1 and 3), or infected for 6 h with influenza A virus (A, lanes 2 and 4). Northern analysis was used to detect either p56 mRNA (lanes 1 and 2) or ISG15 mRNA (lanes 3 and 4).
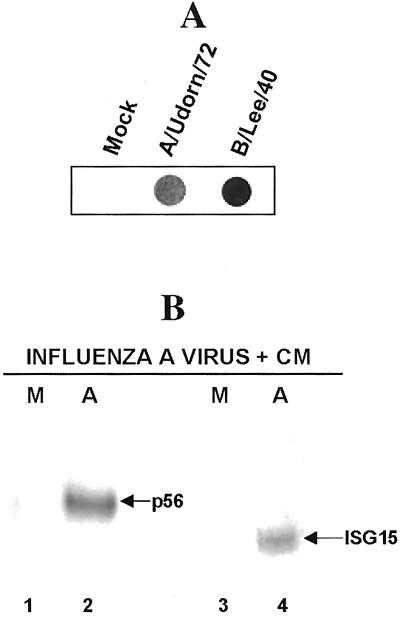
At Least One Influenza A Virus-Encoded Protein Mediates the Inhibition of the Production of Mature mRNAs Encoded by ISRE-Controlled Cellular Genes.
Previous work has established that the inhibition of the posttranscriptional processing of newly synthesized cellular premRNAs is mediated by the NS1A protein (26–29). Hence, it would be predicted that this posttranscriptional block would not occur in the absence of viral protein synthesis and that mature antiviral mRNAs would be produced. To determine whether this prediction is the case, we infected GRE cells with influenza A virus in the presence of CM. Under these conditions, mature p56 and ISG15 mRNAs are produced (Fig. 4B), indicating that at least one influenza A virus-encoded protein, presumably the NS1A protein, mediates the inhibition of the posttranscriptional processing of p56 and ISG15 premRNAs.
The Virus-Specific Events That Are Required for the Activation of IRF-3 Differ Between Influenza A and Influenza B Virus.
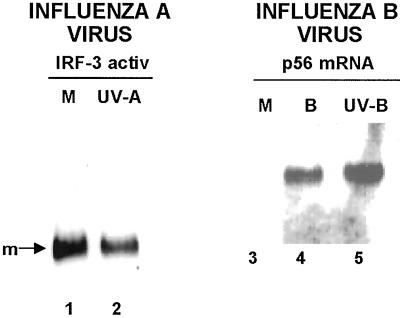
Because influenza virions, like those of other negative-strand RNA viruses, contain the viral RNA polymerase that catalyzes viral mRNA synthesis, transcription catalyzed by this polymerase will take place in the presence of CM (22). To determine whether IRF-3 activation requires such viral RNA transcription, influenza A or influenza B virus was irradiated with 360 mJ of UV light, thereby blocking the virion-associated polymerase from catalyzing transcription. Plaque-forming units per milliliter were reduced from 3–5 × 107 to approximately 102, whereas the hemagglutinin titer was not reduced, suggesting that the binding of the UV-irradiated virus to its cell receptors was not impaired. In cells infected with UV-irradiated influenza A or B virus (at the same level of hemagglutinin units per cell as used with unirradiated virus), no virus-specific protein synthesis was detected (data not shown).
In GRE cells infected with UV-irradiated influenza A virus, the formation of IRF-3 dimers was not detectable (Fig. 5, lanes 1 and 2), indicating that events after the interaction of influenza A virus with the cell surface, most likely viral mRNA synthesis catalyzed by the virion-associated polymerase, are needed for IRF-3 activation. In contrast, UV-irradiated influenza B virus induces essentially the same amount of p56 mRNA in GRE cells as unirradiated influenza B virus (Fig. 5, lanes 4 and 5), indicating that efficient IRF-3 activation and assembly of a functional virus-activated factor is independent of viral mRNA synthesis catalyzed by the virion-associated polymerase.
Figure 5.
Prior UV irradiation blocks the ability of influenza A virus, but not influenza B virus, to activate IRF-3. GRE cells were mock-infected (lanes 1 and 3), or infected for 4 h with UV-irradiated influenza A virus (UV-A, lane 2), unirradiated influenza B virus (B, lane 4), or UV-irradiated influenza B virus (UV-B, lane 5). The cell extracts from lanes 1 and 2 were analyzed for IRF-3 dimerization, and the cell extracts from lanes 3–5 were subjected to Northern blot analysis to detect p56 mRNA.
Discussion
In this paper, we have examined the IFN-α/β-independent activation of cellular transcription that constitutes an early antiviral response of cells against influenza A and B viruses. We show that influenza B virus induces the synthesis in human cells of several mature mRNAs encoded by genes containing an ISRE, thereby establishing that the IRF-3 transcription factor, which is required for the transcription of ISRE-controlled genes, is activated after influenza B virus infection. The production of these cellular mRNAs is independent of both IFN-α/β and viral protein synthesis. In contrast, after infection by influenza A virus, these mature cellular antiviral mRNAs are not produced. However, using a nuclear runoff transcription assay, we establish that the transcription of the ISRE-controlled p56 gene is activated after infection by influenza A virus. Consequently, we conclude that the absence of mature cellular mRNAs encoded by these cellular genes results from the inhibition of the posttranscriptional processing of the premRNAs transcribed from these genes, as is the case for other newly synthesized cellular premRNAs in influenza A virus-infected cells (26–29). In addition, the nuclear runoff transcription assay, in confirmation of two other assays for the activation of IRF-3, establishes that IRF-3 is activated after influenza A virus infection to function in the transcription of ISRE-controlled cellular genes. Further, we show that IRF-3 activation in influenza A virus-infected cells is independent of both IFN-α/β and the synthesis of virus-encoded proteins.
Some of the cellular proteins that are induced in influenza virus-infected cells inhibit virus replication, indicating that the early IFN-α/β-independent activation of transcription constitutes an initial antiviral response of cells against human influenza viruses. Our results, coupled with those obtained with other mammalian viruses (4–6, 11–14, 17), suggest that such an early IFN-α/β-independent activation of the transcription of cellular antiviral genes may be a feature common to many, if not most, mammalian viruses. Influenza A virus is unique in that this activation of cellular transcription does not result in the production of mature cellular mRNAs. As a result, this early antiviral response of cells is effectively countered because the antiviral proteins encoded by these cellular genes are not synthesized. For example, we have shown that 2–5 OAS, an inhibitor of virus replication (36), is not synthesized after influenza A virus infection, so that it is unnecessary for the NS1A protein to inhibit 2–5 OAS activation by sequestering dsRNA. The latter role for the NS1A protein has been proposed by others (38–40). The global inhibition of the early, IFN-α/β-independent cellular antiviral response by influenza A virus may be an important factor in the pathogenicity of influenza A virus infection. In contrast, these cellular antiviral proteins are synthesized in human cells infected by influenza B virus. In addition, mature antiviral mRNAs and their encoded proteins are synthesized in human cells infected by another mammalian virus, cytomegalovirus (3, 7, 9, 11, 12). Consequently, for influenza B virus and cytomegalovirus to replicate, subsequent viral countermeasures against this early cellular antiviral response would likely be needed. It is not known how influenza B virus counters the early IFN-α/β-independent antiviral response of cells.
Previous work has established that the nuclear export of newly synthesized cellular mRNAs is inhibited in influenza A virus-infected cells, and that this inhibition is mediated by the viral NS1A protein by way of its inhibition of the 3′ end processing of newly synthesized cellular premRNAs (26–29). However, it was not known how this function of the NS1A protein benefits influenza A virus replication. On the basis of the results of the present paper, we conclude that one of the major roles of the NS1A protein-mediated inhibition of the processing of newly synthesized cellular premRNAs is the inhibition of the early, IFN-α/β-independent production of cellular antiviral mRNAs. In fact, we have found that a recombinant A/Udorn/72 virus that encodes a NS1A protein with a point mutation that eliminates the binding of the 30-kDa subunit of the cleavage and polyadenylation specificity factor is highly attenuated, and that mature antiviral mRNAs are produced after infection (D. L. Noah, Y.-Y. Twu, M. Takeda, and R.M.K., unpublished results). Because the NS1B protein of influenza B virus does not inhibit the 3′ end processing of cellular premRNAs and the nuclear export of cellular mRNAs (25), the efficient production of functional cellular antiviral mRNAs occurs in influenza B virus-infected cells.
CM does not inhibit the activation of IRF-3 in cells infected by influenza A or B virus, indicating that the synthesis of influenza A or B virus-encoded proteins is not required for this activation. However, influenza A virus does not activate IRF-3 when transcription by the virion-associated polymerase is blocked by prior UV irradiation of the virus. Such viral transcription may generate virus-encoded, double-stranded RNA molecules, which have been postulated to be responsible for the activation of IRF-3 in cells infected by other negative-strand RNA viruses (1, 2, 5, 6, 8, 10, 13). In contrast, influenza B virus does not require transcription by the virion-associated polymerase, as shown by the efficient induction of antiviral mRNAs by UV-irradiated influenza B virus. Thus, influenza B virus may be similar to two DNA viruses, cytomegalovirus and herpes simplex virus, which activate the transcription of ISRE-controlled genes by an initial interaction of the virus with the cell surface, either the binding of virions to receptors on the cell surface or the subsequent entry of virions into the cell (7, 9, 11, 12).
IFN was discovered in 1957 by using influenza A virus (41), and since that time the production of IFN-α/β resulting from influenza A virus infection has been extensively documented, e.g., in several recent publications (42–44). Consequently, it would be expected that activation of IRF-3, which is required for the transcription of IFN-α/β genes (5, 17), occurs in influenza A virus-infected cells. However, one group of investigators has reported that IRF-3 is not activated during influenza A virus infection and that, as a consequence, IFN-β mRNA is not made (20). These results are in conflict with the evidence from many investigators, who have shown that IRF-3 is activated (present study) and that IFN-α/β is produced (41–44) in cells infected by influenza A virus.
A substantial body of evidence has established that the first cellular defenses against infection by mammalian viruses, including human influenza viruses, take place in initially infected cells before the synthesis of IFN-α/β (4–6, 11–14, 17; present study). In cells initially infected with influenza A virus these IFN-α/β-independent antiviral responses include not only the IFN-α/β-independent activation of the transcription of antiviral genes (present study) but also the activation of constitutively expressed protein kinase R (45, 46). An interplay between cellular antiviral responses and influenza A viral countermeasures would then ensue. One such countermeasure, the posttranscriptional inhibition of the production of mature cellular antiviral mRNAs, is reported in the present study. The eventual outcome of the virus–cell battle in human cells initially infected with influenza A viruses is the production of IFN-α/β (41–44).
Acknowledgments
We thank Saumen Sarkar and Ganes Sen (The Cleveland Clinic) for kindly providing the p56 and 2–5 OAS antisera and for help in Western blotting with the 2–5 OAS antiserum. We thank Arthur Haas (Medical College of Wisconsin, Milwaukee) for providing the ISG15 antiserum and Peter Howley (Harvard Medical School, Boston) for providing the SL-12 anti-IRF-3 monoclonal antibody. We thank Weiming Yuan and Diana Noah for helpful discussions. This investigation was supported by National Institutes of Health Grant AI11772 (to R.M.K.).
Abbreviations
- IRF-3
IFN regulatory factor-3
- ISRE
IFNα/β-stimulated response elements
- CBP
CREB (cAMP-responsive element binding protein)-binding protein
- 2–5 OAS
2′-5′ oligo(A) synthetase
- CM
cycloheximide
References
- 1.Daly C, Reich N C. Mol Cell Biol. 1993;13:3756–3764. doi: 10.1128/mcb.13.6.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly C, Reich N C. J Biol Chem. 1995;270:23739–23746. doi: 10.1074/jbc.270.40.23739. [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Cong J P, Shenk T. Proc Natl Acad Sci USA. 1997;94:13985–13990. doi: 10.1073/pnas.94.25.13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarro L, Mowen K, Rodems S, Weaver B, Reich N, Spector D, David M. Mol Cell Biol. 1998;18:3796–3802. doi: 10.1128/mcb.18.7.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wathelet M G, Lin C H, Parekh B S, Ronco L V, Howley P M, Maniatis T. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 6.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle K A, Pietropaolo R L, Compton T. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Peters K L, Sen G C. Virology. 2000;267:209–219. doi: 10.1006/viro.1999.0135. [DOI] [PubMed] [Google Scholar]
- 9.Chin K C, Cresswell P. Proc Natl Acad Sci USA. 2001;98:15125–15130. doi: 10.1073/pnas.011593298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K, Okabe Y, Namiki H, Fujita T. Genes Cells. 2001;6:375–388. doi: 10.1046/j.1365-2443.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- 11.Preston C M, Harman A N, Nicholl M J. J Virol. 2001;75:8909–8916. doi: 10.1128/JVI.75.19.8909-8916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmen K A, Singh J, Luukkonen B G, Lopper M, Bittner A, Miller N E, Jackson M R, Compton T, Fruh K. Proc Natl Acad Sci USA. 2001;98:7140–7145. doi: 10.1073/pnas.121177598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver B K, Kumar K P, Reich N C. Mol Cell Biol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wathelet M G, Berr P M, Huez G A. Eur J Biochem. 1992;206:901–910. doi: 10.1111/j.1432-1033.1992.tb16999.x. [DOI] [PubMed] [Google Scholar]
- 15.Biron C A, Sen G C. In: Fields Virology. 4th Ed. Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 2001. pp. 321–351. [Google Scholar]
- 16.Thanos D, Maniatis T. Cell. 1992;71:777–789. doi: 10.1016/0092-8674(92)90554-p. [DOI] [PubMed] [Google Scholar]
- 17.Maniatis T, Falvo J V, Kim T H, Kim T K, Lin C H, Parekh B S, Wathelet M G. Cold Spring Harbor Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- 18.Yie J, Merika M, Munshi N, Chen G, Thanos D. EMBO J. 1999;18:3074–3089. doi: 10.1093/emboj/18.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darnell J E, Jr, Kerr I M, Stark G R. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 20.Talon J, Horvath C M, Polley R, Basler C F, Muster T, Palese P, Garcia-Sastre A. J Virol. 2000;74:7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan W, Krug R M. EMBO J. 2001;20:362–371. doi: 10.1093/emboj/20.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb R A, Krug R M. In: Fields Virology. 4th Ed. Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 2001. pp. 1487–1532. [Google Scholar]
- 23.Wright P F, Webster R G. In: Fields Virology. 4th Ed. Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 2001. pp. 1533–1579. [Google Scholar]
- 24.Osterhaus A D, Rimmelzwaan G F, Martina B E, Bestebroer T M, Fouchier R A. Science. 2000;288:1051–1053. doi: 10.1126/science.288.5468.1051. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Krug R M. Virology. 1996;223:41–50. doi: 10.1006/viro.1996.0453. [DOI] [PubMed] [Google Scholar]
- 26.Nemeroff M, Barabino S M L, Keller W, Krug R M. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Li Y, Krug R M. EMBO J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Krug R M. Trends Microbiol. 2000;8:376–383. doi: 10.1016/s0966-842x(00)01794-7. [DOI] [PubMed] [Google Scholar]
- 29.Shimuzu K, Iguchi A, Gomyou R, Ono Y. Virolology. 1999;254:213–219. doi: 10.1006/viro.1998.9555. [DOI] [PubMed] [Google Scholar]
- 30.Katze M G, Krug R M. Mol Cell Biol. 1984;4:2198–2206. doi: 10.1128/mcb.4.10.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosh A, Desai S Y, Sarkar S N, Ramaraj P, Ghosh S K, Bandyopadhyay S, Sen G C. J Biol Chem. 1997;272:15452–15458. doi: 10.1074/jbc.272.24.15452. [DOI] [PubMed] [Google Scholar]
- 32.Loeb K R, Haas A L. J Biol Chem. 1992;267:7806–7813. [PubMed] [Google Scholar]
- 33.Guo J, Hui D J, Merrick W C, Sen G C. EMBO J. 2000;19:6891–6899. doi: 10.1093/emboj/19.24.6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronco L V, Karpova A Y, Vidal M, Howley P M. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 36.Silverman R H. J Interferon Res. 1994;14:101–104. doi: 10.1089/jir.1994.14.101. [DOI] [PubMed] [Google Scholar]
- 37.Bandyopadhyay S K, Leonard G T, Jr, Bandyopadhyay T, Stark G R, Sen G C. J Biol Chem. 1995;270:19624–19629. doi: 10.1074/jbc.270.33.19624. [DOI] [PubMed] [Google Scholar]
- 38.Basler C F, Reid A H, Dybing J K, Janczewski T A, Fanning T G, Zheng H, Salvatore M, Perdue M L, Swayne D E, Garcia-Sastre A, et al. Proc Natl Acad Sci USA. 2001;98:2746–2751. doi: 10.1073/pnas.031575198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Sastre A. Virology. 2001;279:375–384. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- 40.Salvatore M, Basler C F, Parisien J P, Horvath C M, Bourmakina S, Zheng H, Muster T, Palese P, Garcia-Sastre A. J Virol. 2002;76:1206–1212. doi: 10.1128/JVI.76.3.1206-1212.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Issacs A, Lindemann J. Proc R Soc Lond Ser B. 1957;147:258–267. [PubMed] [Google Scholar]
- 42.Ronni T, Matikainen S, Sareneva T, Melen K, Pirhonen J, Keskinen P, Julkunen I. J Immunol. 1997;158:2363–2374. [PubMed] [Google Scholar]
- 43.Huang Q, Liu D, Majewski P, Schultele A C, Korn J M, Young R A, Lander E S, Hacohen N. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 44.Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Cytokine Growth Factor Rev. 2001;12:171–180. doi: 10.1016/s1359-6101(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 45.Hatada E, Saito S, Fukuda R. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergmann M, Garcia-Sastre A, Carnero E, Pehamberger H, Wolff K, Palese P, Muster T. J Virol. 2000;74:6203–6206. doi: 10.1128/jvi.74.13.6203-6206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]